The amount of electricity consumed worldwide grows by the year, and so does the demand for energy storage solutions since many devices often operate in autonomous mode. Rechargeable lithium-ion batteries have been workhorse of the consumer electronics market including portable electronics, implantable devices, power tools, hybrid/full electric vehicles (EVs) and global power grids, due to their ability to store large amounts of energy per unit weight and per unit volume, fairly high discharge and charge rates and low self-discharge rate, and long cycle life. For instance, Australia is launching a series of large-scale lithium-ion battery storage projects to manage excess solar and wind energy. The need for high‐performance and low‐cost batteries is driven by the growing market of electromobility, in order to fulfill key requirements, such as a sufficient driving range and fast charging ability, for achieving broad consumer acceptance.
Big technology and car companies are all too aware of the limitations of lithium-ion batteries. While chips and operating systems are becoming more efficient to save power we’re still only looking at a day or two of use on a smartphone before having to recharge. In addition, Lithium-ion batteries use toxic, heavy metals, such as lithium and cobalt, which can negatively impact the environment when they are extracted as well as disposed.
Another challenge is material avilability, If lithium-ion batteries continue to be produced in growing quantities, the world may sooner or later run out of lithium reserves. With Congo producing 60% of cobalt for lithium-ion batteries’ cathodes, cobalt prices may skyrocket. The same goes for lithium, as lithium mining’s water consumption poses a great challenge for the environment. Therefore, researchers are looking for new energy storage devices relying on more accessible materials while using the same operating principle as lithium-ion batteries.
Global warming, fossil fuel availability and energy management challenges are leading the way towards sustainable energy production methods such as electrical energy production such as Wind and hydropower turbines, photovoltaic panels, biomass conversion and geothermal power plants. However energy storage technologies are lagging in this trend. Electrochemical energy storage systems (EESS) (e.g. batteries and supercapacitors) have thus become essential elements towards a sustainable energy management economy. The ever-increasing demand for transportation and consumer electronics mobility further adds to the strategic importance of EESS.
The main challenges to overcome for EESS to really enable this revolution can be resumed to three words – Cost, Abundance and Reliability
(CAR) . Making a battery at lowest costs, based on abundant elements and resources and with a long cycle life has become the challenging
dream of any battery scientist and engineer. Low costs should be associated to any aspect related to battery life cycle – raw materials, production, assembly, use and end-of-life disposable (here, ideally, through efficient recycling). Closely related to raw materials costs, abundance is also an important element. Since the stored energy (or the stored charge) scales linearly with the mass of the active battery materials, massive production of batteries will inevitably require enormous amounts of raw materials (e.g. lithium, cobalt, nickel following current technology) so that developing chemistries based on scarce elements is not justified. Finally, a long and safe cycle life (ideally, more than 25 years of unfaulty operation or equivalent of 10.000 recharges) is highly sought.
![PDF] Organic Batteries-the route towards sustainable electrical energy storage technologies Organic Batteries-the route towards sustainable electrical energy storage technologies | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0f56722980ec309778426e0edd3e322d78eca514/3-Figure1-1.png)
Thus, to truly promote low emission electrochemical energy storage systems, a possible alternative would be in partially moving away from inorganic-based to allorganic-based electroactive electrode materials. Indeed, organic materials are based on naturally abundant elements (e.g. C, H, O, N, S) coupled with the real possibility of being generated from renewable resources (biomass). In addition, organic materials are typical fuels that can be easily burned by simple thermal combustion at medium temperatures making readily possible the recyclability of constituent elements via CO2 valorisation. All of these advantages offer new possibilities for low cost, greener and sustainable energy storage devices.
The concept vehicle of Mercedes-Benz designers, ADVANCED VEHICLE TRANSFORMATION for mobility in the distant future is based on organic battery. The VISION AVTR was designed in line with its innovative electric drive. This is based on a particularly powerful and compact high-voltage battery. For the first time, the revolutionary battery technology is based on graphene-based organic cell chemistry and thus completely eliminates rare, toxic and expensive earths such as metals. Electromobility thus becomes independent of fossil resources. An absolute revolution is also the recyclability by composting, which is 100% recyclable due to the materiality. As a result, Mercedes-Benz underlines the high relevance of a future circular economy in the raw materials sector

Environmentally speaking, the richness of organic chemistry allows us to design green battery materials from renewable starting compounds through low environmental footprint processes. The potential organic battery materials can be generated from bio-sources via eco-friendly synthetic processes. The dilithium rhodizonate may be obtained from the natural compound myo-inositol that can be found in the majority of plants in the form of phytic acid. Note that dilithium rhodizonate is a competitive electrode material that can provides high specific and volumetric energy densities, of 1100 W.h.kg-1 and 2400 W.h.L-1 respectively
Recent Progress
A alternative technology is in its infancy, but researchers think bacteria and paper may provide a way of creating more sustainable batteries. IEEE Spectrum reports that research by Seokheun Choi and colleagues at the State University of New York at Binghamton “focuses on integrating bacteria into paper both to generate electricity and to dispose of the battery…To create the battery, the research team placed freeze-dried “exoelectrogens” on paper. They explain that exoelectrogens are a type of bacteria that can transfer electrons outside of their cells. The electrons pass through the cell membrane and make contact with external electrodes to power the battery…To activate the battery, the researchers added water or saliva, both of which revived the bacteria”. At the end of its lifecycle, the “hybrid paper-polymer biobattery readily decomposes in water.”
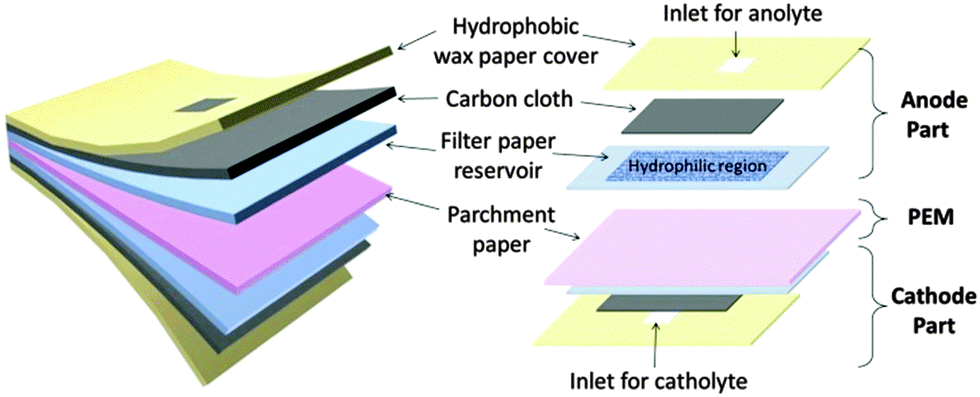
Researchers in Spain are working on “the PowerPAD (Power: Portable And Disposable), a fully organic and completely biodegradable battery concept inspired by the sustainability principles of green electronics. [It} represents a new class of batteries designed to operate for relatively short periods of time (from minutes to 1–2 h) to fulfill the power needs of portable applications while not requiring any specific recycling facility for its disposal…The two electrodes made of hydrophilic porous carbon paper are… sandwiched between two thin layers of cellulose in order to ensure reliable capillary flow of reactants through the electrodes and into the large cellulose absorbent pad at the bottom. The outward facing surfaces of the device are sealed with a beeswax-covered cellulose layer in order to prevent liquid leakages…The proposed battery is conceived as a single use power source that is activated by the addition of a small sample of liquid, such as water, urine, or saliva, on the inlet cellulose pad.”
Researchers at Texas A&M University under guidance of Dr Jodie L. Lutkenhaus are making progress with 100% polymer batteries. Appearing in Nature Materials as quoted in Science Daily she says these will recycle faster than traditional batteries. Her team has confirmed organic radical polymers appear ideal in terms of electrochemical activity too. “These polymers are very promising for batteries because they can charge and discharge way faster than any common battery,” she explains. ”This rapid charging could dramatically change the way electric vehicles (and phones) are used today.” We understand the chemical structures are also ‘very stable and reactive”. That’s because they have a single electron in the radical group. Moreover, this unpaired electron allows rapid charge transfer in these polymers during redox reactions. However, Dr Lutkenhaus has only observed this phenomenon under laboratory conditions. She hence needs more time to research organic radical batteries in order to understand them better
An All-Organic Proton Battery Charges In Seconds
Researchers at Uppsala University have therefore developed an all-organic proton battery that can be charged in a matter of seconds. The battery can be charged and discharged over 500 times without any significant loss of capacity. Their work has been published in the scientific journal Angewandte Chemie. The researchers have been able to demonstrate that their battery can be easily charged using a solar cell. Charging can also be accomplished without the aid of the advanced electronics that, for example, lithium batteries require. Another advantage of the battery is that it is unaffected by ambient temperature.

“I’m sure that many people are aware that the performance of standard batteries declines at low temperatures. We have demonstrated that this organic proton battery retains properties such as capacity down to as low as -24°C,” says Christian Strietzel of Uppsala University’s Department of Materials Science and Engineering. A great many of the batteries manufactured today have a major environmental impact, not least due to the mining of the metals used in them.
“The point of departure for our research has therefore been to develop a battery built from elements commonly found in nature and that can be used to create organic battery materials,” explains Christian Strietzel. For this reason, the research team has chosen quinones as the active material in their battery. These organic carbon compounds are plentiful in nature, among other things occurring in photosynthesis. The characteristic of quinones that researchers have utilised is their ability to absorb or emit hydrogen ions, which of course only contain protons, during charging and discharging.
An acidic aqueous solution has been used as an electrolyte, the vital component that transports ions inside the battery. As well as being environmentally friendly, this also provides a safe battery free from the hazard of explosion or fire. “There remains a great deal of further development to be done on the battery before it becomes a household item; however, the proton battery we have developed is a large stride towards being able to manufacture sustainable organic batteries in future,” says Christian Strietzel.
Russian chemists developed polymer cathodes for ultrafast batteries reported in Jan 2021
Russian researchers from Skoltech, D. Mendeleev University, and the Institute of Problems of Chemical Physics of RAS have synthesized and tested new polymer-based cathode materials for lithium dual-ion batteries. The tests showed that the new cathodes withstand up to 25,000 operating cycles and charge in a matter of seconds, thus outperforming lithium-ion batteries. The cathodes can also be used to produce less expensive potassium dual-ion batteries. The research was published in the journal Energy Technology.
The team used a promising post-lithium dual-ion technology based on the electrochemical processes involving the electrolyte’s anions and cations to attain a manifold increase in lithium-ion batteries’ charging rate. Another plus is that the cathode prototypes were made of polymeric aromatic amines synthesized from various organic compounds.
“Our previous research addressed polymer cathodes for ultra-fast high-capacity batteries that can be charged and discharged in a few seconds, but we wanted more,” says Filipp A. Obrezkov, a Skoltech Ph.D. student and the first author of the paper. “We used various alternatives, including linear polymers, in which each monomeric unit bonds with two neighbors only. In this study, we went on to study new branched polymers where each unit bonds with at least three other units. Together they form large mesh structures that ensure faster kinetics of the electrode processes. Electrodes made of these materials display even higher charge and discharge rates.”
A standard lithium-ion cell is filled with lithium-containing electrolyte and divided into the anode and the cathode by a separator. In a charged battery, the majority of lithium atoms are incorporated in the anode’s crystal structure. As the battery discharges, lithium atoms move from the anode to the cathode through the separator. The Russian team studied the dual-ion batteries in which the electrochemical processes involved the electrolyte’s cations (i.e., lithium cations) and anions that get in and out of the anode and cathode material’s structures, respectively. Another novel feature is that, in some experiments, the scientists used potassium electrolytes instead of expensive lithium ones to obtain potassium dual-ion batteries.
The team synthesized two novel copolymers of dihydrophenazine with diphenylamine (PDPAPZ) and phenothiazine (PPTZPZ), which they used to produce cathodes. As anodes, they used metallic lithium and potassium. Since the cathode drives the key features of these battery prototypes called half-cells, the scientists assemble them to assess the capabilities of new cathode materials quickly.
While PPTZPZ half-cells showed average performance, PDPAPZ turned out to be more efficient: lithium half-cells with PDPAPZ were fairly quick to charge and discharge while displaying good stability and retaining up to a third of their capacity even after 25,000 operating cycles. If a regular phone battery were as stable, it could be charged and discharged daily for 70 years. PDPAPZ potassium half-cells exhibited a high energy density of 398 Wh/kg. For comparison, the value for common lithium cells is 200-250 Wh/kg, the anode and electrolyte weights included. Thus the Russian team demonstrated that polymer cathode materials could create efficient lithium and potassium dual-ion batteries.
Hybrid Batteries
Despite the exciting opportunities and promises, the current organic battery materials and technologies are still far from being ready to challenge the inorganic based LIBs. At best, an all-organic LIB will deliver a voltage of 2 V whereas inorganic systems operate already above 4 V. Gravimetric capacities may be comparable yet the volumetric energy metrics remain poorly competitive. The organic battery field is still in its early stages and there is large room for improvements in terms of gravimetric capacity, redox potential, cycling as well as air stability. And if the perfect chemistry is found here, the synthesis method should then be optimized to achieve this in only a few steps (less than 3 ideally to limit the costs) and proceed through sustainable processes (solvent free, abundant catalysts and co-reactants).
Now, researchers from York University have discovered a way to make lithium-powered batteries more environmentally friendly, while retaining performance, stability and storage capacity. Their latest breakthrough is the creation of a new carbon-based organic molecule that can replace the cobalt now used in cathodes found in in lithium-ion batteries. The new material addresses the shortcomings of the inorganic material while still maintaining performance. “Electrodes made with organic materials can make large-scale manufacturing, recycling, or disposing of these elements more environmentally friendly,” says Baumgartner. “The goal is to create sustainable batteries that are stable and have equally as good if not better capacity.
“With this particular class of molecules that we’ve made, the electroactive component is very suitable for batteries as it’s very good at storing electrical charges and has good long-term stability.” Baumgartner and his group previously reported on the electroactive component in a paper published in the journal Advanced Energy Materials. “We have optimized this electroactive component and put it in a battery. It has a very good voltage, up to the 3.5 volts, which is really where current batteries are now,” he says. “It’s an important step forward in making fully organic and sustainable batteries.”
Baumgartner, along with postdoctoral researchers Colin Brides and Monika Stolar, have also demonstrated that this material is stable in long-term operation with the ability to charge and discharge for 500 cycles. One of the downsides of inorganic electrodes is that they generate significant heat when charging and require limited discharging rates for safety reasons; this new molecule addresses that shortcoming. The next step, says Baumgartner, is to improve the capacity further. His team is currently developing the next generation of molecules that show promise in being able to increase current capacity.
Yet the potential of organic batteries is still to be uncovered and there is great scientific and technological potential behind. Organic chemistry is versatile and can offer access to different chemical functionalities so that the capacity, voltage, solubility and the reaction
kinetics could be tuned at will. Recycling routes may be also more efficient, since the organic materials could be derived from biomass and
combusted at medium temperatures. Finally, organic battery materials are less sensitive to the cation metal chemistry so that it can easily
allow switching form Li-ion technology towards Na, Mg and Ca based batteries.
Referencies and Resources also include:
https://www.eurekalert.org/pub_releases/2021-01/sios-rcd011921.php
 International Defense Security & Technology Your trusted Source for News, Research and Analysis
International Defense Security & Technology Your trusted Source for News, Research and Analysis