Since December 31, 2019, when the 2019-nCoV coronavirus was first reported from Wuhan, China, It has spread like wildfire to every corner of the globe, posing a serious threat for the foreseeable future. Millions have been infected, although a worse tragedy has been avoided thanks to widespread adoption of social distancing, public closures, and practices like handwashing and mask-wearing.
As the ongoing coronavirus outbreak centered in China has spread to other countries and been blamed for a growing number of deaths, a consensus has emerged that this particular virus, currently classified as a “novel [i.e. new] coronavirus,” is believed to have originated in bats and was transmitted to humans in Wuhan, China via a seafood market that also traded exotic animals. So-called “wet” markets, like the one in Wuhan, were previously blamed for past deadly coronavirus outbreaks in China, such as the 2003 outbreak of Severe Acute Respiratory Syndrome (SARS). Viruses that have caused past pandemics typically originated from animal influenza viruses. Zoonotic disease outbreaks occur when a virus, bacterium, or fungus jumps from an animal to a human.
Pandemics put millions of lives are at risk and their economic consequences can run into billions. The 1918 pandemic killed approximately 50 million people around the globe, making it one of the deadliest events in human history. Last Ebola outbreak cost more than 11,000 human lives and more than $32 billion in economic ripple effects while Zika has cost the economies of Latin America and the Caribbean an estimated $18 billion. Eliminating pandemic outbreaks and mitigating the impact of a potential high threat biological agent release are national security priorities.
Pandemics also affect militaries. Warfighters must also operate in regions where diseases like chikungunya and dengue are endemic, and even seemingly mild challenges like seasonal influenza affect force readiness. Military service members are called upon to operate virtually anywhere in the world, often on short notice, and the locations to which they deploy frequently lack the robust public health infrastructure to identify and contain the spread of new viral infectious diseases. With coronavirus outbreak, synthetic biologists are applying cutting-edge tools and technology to help responders go from detection to cure with unprecedented speed and scale.
Media outlets have also linked the coronavirus outbreak to Biowarfare programs. Several media outlets have promoted claims that the reported epicenter of the outbreak in Wuhan, China was also the site of laboratories allegedly linked to a Chinese government biowarfare program. Earler some media reports expressed the fear that bats could be used as biological weapons, particularly in spreading coronaviruses and other deadly diseases. The Washington Post asserted that the Pentagon’s interest in investigating the potential use of bats to spread weaponized and deadly diseases was because of alleged Russian efforts to do the same. However, those claims regarding this Russian interest in using bats as bioweapons date back to the 1980s when the Soviet Union engaged in covert research involving the Marburg virus, research that did not even involve bats and which ended with the Soviet Union’s collapse in 1991.
Maintaining active disease surveillance around the world is costly and time-consuming, accurate prediction of when and where these infections will occur next can enable us to better mitigate these outbreaks before they become epidemics. On January 2018, the Defense Advanced Research Projects Agency (“DARPA”) issued a new solicitation in the form of a broad agency announcement for the Preventing Emerging Pathogenic Threats—or “PREEMPT”—program. PREEMPT, seeks to support military readiness by going after new viral infectious diseases at the source, animal reservoirs—the species in which a pathogen lives, multiplies, and potentially evolves into a strain that can threaten humans. PREEMPT aims to advance understanding of viruses and their interaction with animals, insects, and humans, and deliver new, proactive interventions to reduce the risk from emerging and reemerging pathogens.
Zoonotic Viruses
Some of the worst viral disease outbreaks in recent years — SARS, MERS, Ebola, Marburg and likely the newly arrived 2019-nCoV virus — originated in bats. A new University of California, Berkeley, study finds that bats’ fierce immune response to viruses could drive viruses to replicate faster, so that when they jump to mammals with average immune systems, such as humans, the viruses wreak deadly havoc.
Some bats — including those known to be the original source of human infections — have been shown to host immune systems that are perpetually primed to mount defenses against viruses. Viral infection in these bats leads to a swift response that walls the virus out of cells. While this may protect the bats from getting infected with high viral loads, it encourages these viruses to reproduce more quickly within a host before a defense can be mounted. This makes bats a unique reservoir of rapidly reproducing and highly transmissible viruses. While the bats can tolerate viruses like these, when these bat viruses then move into animals that lack a fast-response immune system, the viruses quickly overwhelm their new hosts, leading to high fatality rates.
Animals host a massive number of viruses, and sometimes these viruses make the jump to humans. (These viruses are called zoonoses.) That’s because there are a lot of hurdles that an animal virus has to clear before it spreads to a person, and from there, to another person. “A virus doesn’t just jump out of a bat and cause an epidemic in humans,” says Ronald Rosenberg, an infectious disease researcher. Instead, a virus can spend decades or even centuries hopping back and forth between animals and humans before the conditions come together for an outbreak. (The exception are influenza viruses, which can make this leap more rapidly, Rosenberg says. )
Some Researchers are concentrating on humans, once a virus has made that rare leap — but before it spreads out of control. What we really need, experts like Lloyd-Smith and Rosenberg say, is better surveillance in human communities — especially in ones that frequently come into contact with wildlife.
That means setting up sentinel clinics in viral hot spot regions that can screen sick patients for the usual infectious suspects. Central laboratory facilities could hunt for less typical, or completely unknown, infections, if those initial screens come up negative. Rosenberg is currently piloting such a system in Uganda.
The World Health Organization has launched a project called the R&D Blueprint, to spur development of countermeasures for the diseases the agency believes pose the most critical risk, including Crimean-Congo hemorrhagic fever.
Preventing pandemics
Using mathematical modeling approaches, scientists have estimated there may be about 1.3 million undiscovered viruses in the world — “plus or minus,” Carroll said. Of those, about a half million may be zoonotic — viruses that can jump from an animal species to infect and spread among people, Mazet said.
And a pilot project that has been underway for the past seven years — called PREDICT — has discovered about 1,000 new viruses. The Global Virome Project, a nascent effort being driven by a community of interested scientists, would be an extension of this type of work. The approach, they believe, may be more critical now than ever.
Planners of the Global Virome Project have estimated it would cost about $3.4 billion to locate and gather at least preliminary information on 99 percent of those unknown viral threats. (The other 1 percent are so rare that setting out to find them too could nearly double the cost of the effort; no one seems to be pushing for that at this point.)
Peter Daszak, an epidemiologist with the research and conservation nonprofit EcoHealth Alliance, wants to find these viruses before they make anyone sick. “If we allow these viruses to get into people, it’s already too late,” he says. To do that, he and his team hunted through the scientific literature to create a database of nearly 600 viruses and the more than 750 mammals they infect. Then, the researchers looked for patterns that could help them understand what makes an animal virus more likely to infect humans. Their research was published this week in the journal Nature.
Using computers for Epidemic prediction
Scientists are racing to find ways to predict which infectious disease might emerge and threaten humans next, which could give us more time to implement prevention strategy. Many strategies are being implemented , some are hunting for viruses in animals, which are likely to infect people.
Barbara A. Han, PhD, disease ecologist at Cary Institute of Ecosystem Studies, Millbrook, New York, and her colleagues built a computer program to analyze a massive database of mammalian habits and habitats, including the geographic range and reproductive strategies for hundreds of species and used it to predict which of the 2,277 existing rodent species will serve as zoonotic disease carriers in the future and where they are likely to spread diseases. Their model considered 86 variables, like body size, life span, and population density, to hunt for patterns common among animals known to carry zoonotic diseases.
Han said in a press release. “We were interested in how machine learning could inform early warning surveillance by revealing the distribution of rodent species that are effective disease reservoirs.” Based on its findings, the team was also able to identify hot spots where a disease was more likely to jump from rodent to humans, Kansas, Nebraska, China, Kazakhstan and parts of the Middle East are potential hotspots.
USC Viterbi School of Engineering Ph.D. students Charalampos Chelmis and Anand Panangadan and computer science Ph.D. candidate Ajitesh Srivastava earn national recognition for forecasting outbreaks. They produced a prediction model that uses information sharing to accurately deliver a six-month forecast of the spread of the Chikungunya virus in 55 different countries and territories in North, Central and South America and the Caribbean.
“Information sharing on the Internet is based off of information sharing on epidemics, so we took that and applied it to predicting outbreaks,” Srivastava said. “It’s a similar method to the process of assessing how a meme or a video goes viral.” DARPA began the CHICKV Challenge in 2014 to create models that could accurately predict the spread of Chikungunya.
Synthetic Biologists race to develop detection, vaccination to cure technologies
Many new rapid tests, therapeutic treatments, and vaccine candidates are on the cusp of approval for a disease that just one year ago didn’t exist. Synthetic biology, is playing great part in this revolution whose rapid design-build-test cycles help innovators go from antibodies to candidate vaccines in as little as 24 hours.
“Covid-19 is an unprecedented challenge,” Cratsenburg Chief Business Officer at Ginkgo Bioworks, says. “By leveraging innovation in biology — including the impressive advances in DNA sequencing and genetic engineering — with industrial automation and advanced computation, synthetic biology is uniquely suited to develop and provide solutions to combat the coronavirus pandemic.”
The 2019-nCoV is difficult to detect during the first two weeks after infection. Infected individuals may not be aware of their contagiousness, thus putting others at risk of contracting the virus. There are cases reported that people can be infectious without showing symptoms. A fast and reliable detection method is needed to help researchers better understand the biology of the disease and potentially guide future diagnostics and treatment. To address this, GenScript is freely offering to researchers a high-tech test for the coronavirus. The test is based on a qRT-PCR detection assay, which uses precise DNA strands to accurately detect and measure the amount of an infectious agent like coronavirus in the bloodstream, for example.
Ginkgo is deploying its platform to rapidly scale-up testing for the virus. Ginkgo is also collaborating with partners to develop and manufacture much-needed vaccines and antibody therapeutics. It has committed $25 million of access to its state-of-the-art platform to support research and development efforts for Covid-19 and is working to provide on-site testing for schools and businesses through Concentric by Ginkgo.
Mammoth Biosciences, a company developing a toolbox for the next generation of CRISPR-based diagnostics, is partnering with UC San Francisco researchers who are developing a diagnostic test to identify people infected with the new coronavirus. As Leah Rosenbaum of Forbes reports, testing for suspected coronavirus now requires shipping samples to the Centers for Disease Control and Prevention, where it can take six or more hours to complete the test. The new test from Mammoth Bioscience will work by taking a sample from a nasal swab, putting it into a tube with the CRISPR-Cas system, and then dipping in a color-changing strip of paper to determine whether the test result is positive or negative. The whole thing should take one or two hours and could be done in a doctor’s office.
In May, Mammoth announced a partnership with GSK to develop a handheld device that will put the accuracy of a molecular lab for SARS-CoV-2 detection in the hands of consumers. Most recently, Mammoth was awarded a Phase II contract from the NIH RADx program to manufacture and scale-up our DETECTR assay for high-throughput COVID testing in laboratories. “We are harnessing the diversity of nature to develop next-generation CRISPR products, with a mission to improve lives by reading and writing the code of life,” said Chen, Chief Technology Officer of Mammoth Biosciences. “Using our CRISPR-based DETECTR platform, Mammoth is developing Covid-19 diagnostic solutions across the continuum of testing.”
The Coalition for Epidemic Preparedness Innovations (CEPI) awarded Inovio Pharmaceuticals received a $9 million grant to develop a vaccine against the new coronavirus (2019-nCoV). They have already demonstrated positive clinical outcomes with their vaccine against MERS-CoV, another coronavirus. Working with Inovio is Twist Bioscience, which will provide DNA synthesis. Kate Broderick, senior vice-president of research and development at Inovio, told the BBC: “Our DNA medicine vaccines are novel in that they use DNA sequences from the virus to target specific parts of the pathogen which we believe the body will mount the strongest response to. We then use the patient’s own cells to become a factory for the vaccine, strengthening the body’s own natural response mechanisms.” Inovio has plans for its vaccine to enter human trials by the early summer.
Abcellera is working to identify antibodies that can neutralize the virus and potentially block its transmission. Since 2018, under the DARPA Pandemic Prevention Platform (P3) program, AbCellera has been developing a “technology platform for pandemic response capable of developing field-ready medical countermeasures within 60 days of isolation of an unknown viral pathogen.” Abcellera told that a key aspect of this work is to “deliver an antibody countermeasure as a nucleic acid vector instead of recombinant purified protein.” In other words, the patient’s own cells will manufacture the therapeutic instead of it being manufactured in a lab outside of the patient. This is a relatively new way to deliver the drug — or more precisely, the genes to make the drug.
IDT is another DNA synthesis company finding itself involved in the very early stages of addressing emerging diseases. As a long-time player in the field, IDT says it was quickly engaged by researchers with interests in both diagnostic assays and vaccine development. IDT has already shipped synthetic genes for use in the pursuit of coronavirus vaccines, as well as customized oligonucleotide probes and primers that will facilitate more sensitive and accurate detection of the Wuhan virus.
There are many other examples of companies working to bring a coronavirus vaccine to market in record time, including Novavax and Johnson & Johnson. In academia, there are countless labs working to understand the Wuhan coronavirus at a basic science level, providing vital knowledge about what makes this coronavirus unique, what traits it shares with its cousins, and how to anticipate the next pandemic.
Synthetic biology-based COVID vaccine
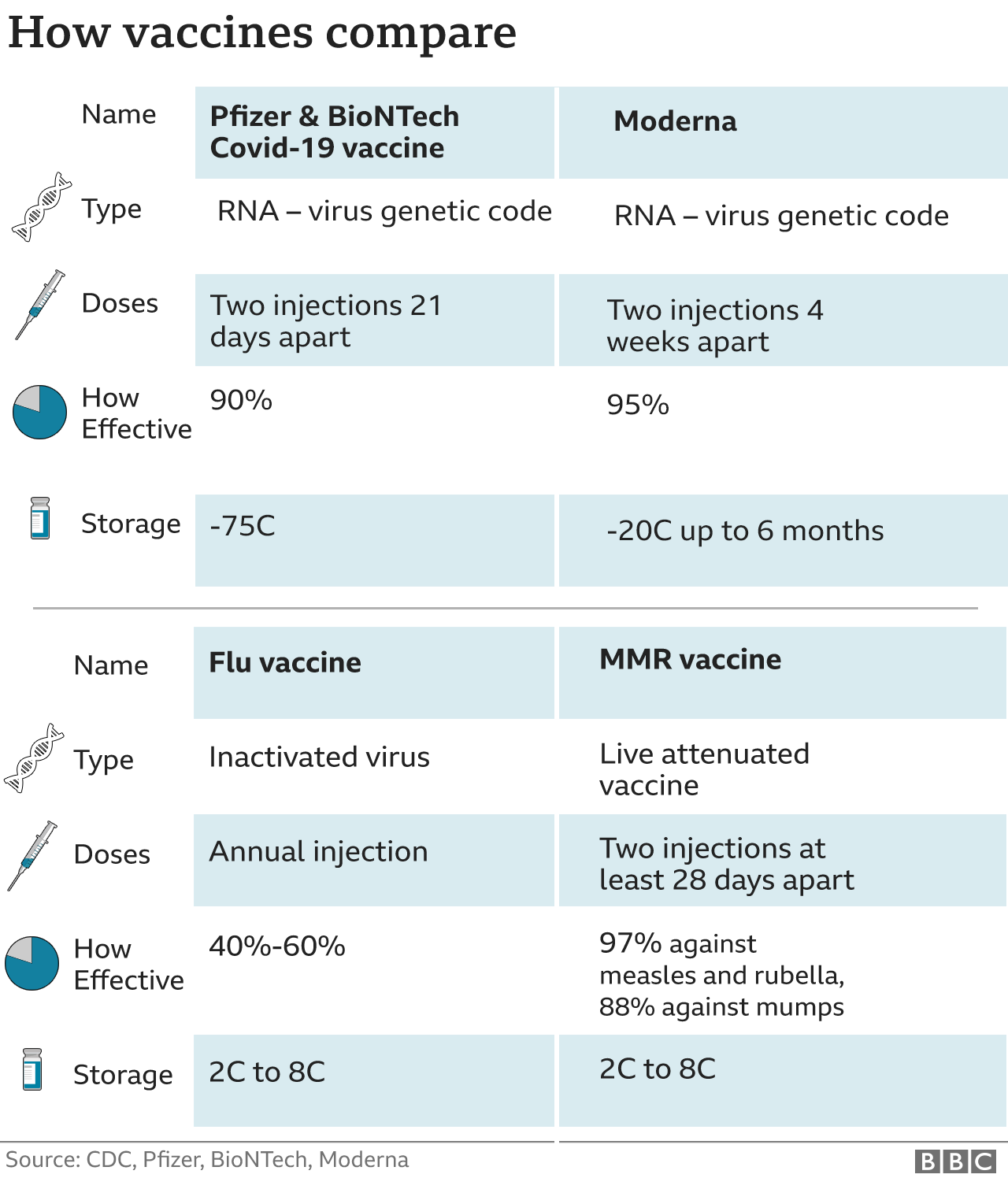
A new vaccine that protects against Covid-19 is nearly 95% effective, early data from US company Moderna shows. The results come hot on the heels of similar results from Pfizer, and add to growing confidence that vaccines can help end the pandemic. Both vaccines use the same approach of injecting part of the virus’s genetic code in order to provoke an immune response. The preliminary data we have seen so far is very similar – around 90% protection for the Pfizer/BioNTech vaccine and around 95% for Moderna’s. However, both trials are still taking place and the final numbers could change. Moderna’s vaccine appears to be easier to store as it remains stable at minus 20C for up to six months and can be kept in a standard fridge for up to a month. Pfizer’s vaccine needs ultra-cold storage at around minus 75C, but it can be kept in the fridge for five days. The Sputnik V vaccine, developed in Russia, has also released very early data which suggests it is 92% effective.
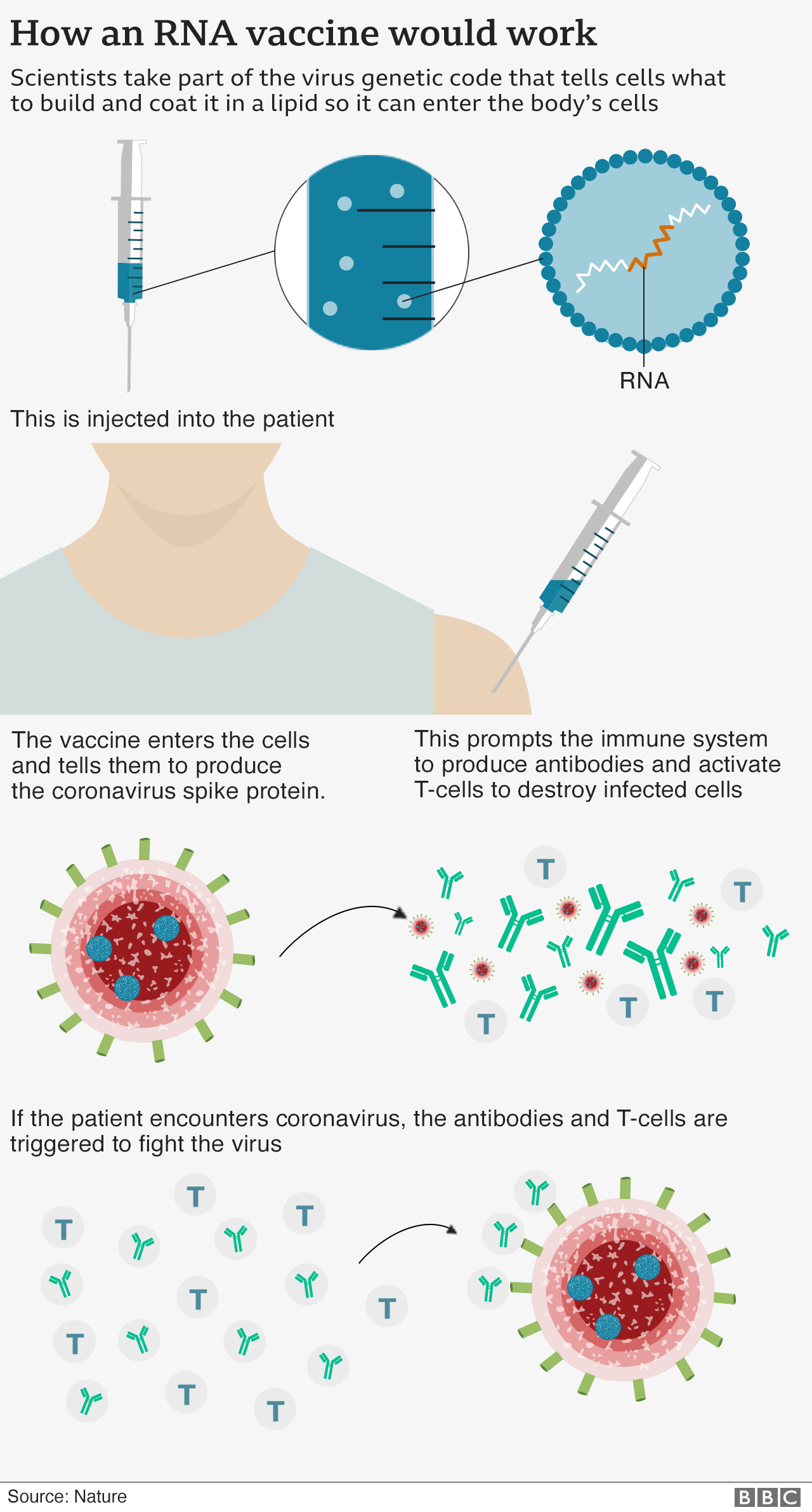
Moderna has developed an “RNA vaccine” – it means part of the coronavirus’s genetic code is injected into the body. This starts making viral proteins, but not the whole virus, which is enough to train the immune system to attack. It should train the body to make both antibodies – and another part of the immune system called T-cells to fight the coronavirus.
Years ago, researchers learned that, when made with biotechnology, some viral proteins could spontaneously assemble themselves into “virus-like particles,” or VLPs. Although benign, these particles looked like a virus and the bodies recognized them as such, producing fantastic immune responses. Gardasil, Merck’s HPV vaccine, was made this way, potentially saving millions of lives over the next century.
[Neil] King figured out how to design similar particles with software, crafting sporty shapes that made them easier to manufacture and, by a geometric sleight-of-hand, potentially far more potent. Last year, he built a candidate for RSV and raised $51 million to launch a company called Icosavax. This year, he turned his attention to the new coronavirus. “We’ve made what looks like a really safe and really effective vaccine,” King told Endpoints News.
The Bill and Melinda Gates Foundation agreed, announcing on [October 2020] that they would pour $10 million into the effort, the most they’ve publicly committed for any early-stage Covid-19 vaccine. The goal is to use synthetic biology to build an exceptionally potent yet easily scalable vaccine, one that can work even in the most at-risk populations, including older adults who might not respond as well to a classic shot. Cell published their preclinical study [November 13], showing high rates of neutralizing antibodies, including from a single dose.
References and resources also incude:
https://synbiobeta.com/the-synthetic-biology-companies-racing-to-fight-coronavirus/
https://www.bbc.com/news/health-54902908
 International Defense Security & Technology Your trusted Source for News, Research and Analysis
International Defense Security & Technology Your trusted Source for News, Research and Analysis