Biophotonic is an interdisciplinary field that covers the interaction between light (electromagnetic radiation) and biological materials such as subcellular structures, cells, tissues, and molecules in living organisms. It holds potential to address important societal challenges, including in human health through the development of medical devices for the management of human diseases; in environmental health by enabling highly sensitive devices for advanced pollution detection; and in food and agriculture through providing means for assessing quality control and safety.
Within medicine and the life sciences, biophotonics promises progress and new developments with regard to a better understanding of the origins of disease, improving diagnosis and follow-up care, preventing disease, and treating patients individually and specifically (‘personalized medicine’). Advances in light-based technologies have resulted in innovative and transformative tools to study and manipulate biological systems at the subcellular, cellular, tissue, and organ levels. Optical devices are used in the clinic today to detect colon tumors, guide surgical excision, perform laser surgery, diagnose dermatological conditions, and far more.
Biophotonics is expanding the scope of radiology by bringing clinicians and researchers new tools for noninvasive imaging of cancer and other diseases. Normal and pathological processes usually present themselves on the macro-and systems-biology levels, but their origins are found at the single-molecule level, which is below the diffraction limit of light. These can take the form of genetic mutations leading to nucleotide base substitution that culminate in uncontrolled tumor growth, or lipid oxidation within the myelin membranes of the neuronal tracts of the spinal cord that cascades to create neurodegenerative disorders like multiple sclerosis and motor neuron disease. Advances in super-resolution imaging, sample handling, and data processing are allowing some of these events to be visualized directly
While the xrays and gamma rays commonly used in imaging represent high energy light sources, biophotonics typically relies on sources at the lower end of the energy spectrum like infrared, near-infrared, visible, and ultraviolet light. This lower energy light helps preserve the biological cells examined even as the optical equipment visualizes structures too small to be seen with Xray, CT, and MRI. Optical biopsies allow the real time detection of abnormal tissue to be studied in the operating room improving the process and helping to avoid the sampling errors common to conventional methods.
“In a conventional biopsy, we take the tissue to the microscope, but with optical biopsy, we’are taking the microscope to the tissue,” said Arthur F. Gmitro, PhD, professor and head of the Department of Biomedical Engineering and professor of medical imaging and optical sciences at the University of Arizona in Tucson. “With an optical biopsy, you scan across the tissue in real-time and make a less invasive and potentially more accurate diagnosis,” Dr. Gmitro said.
Optical coherence tomography (OCT), a high speed, cross-sectional microscopic imaging modality, is another well-established area of biophotonics. Like ultrasound, OCT operates on an echo-based paradigm except that in OCT the ultrasonic waves are replaced by light waves. OCT is commonly used in the eye to study the retina and diagnose glaucoma, macular degeneration and other conditions. “OCT is now used for image guided surgery in the retina and has potential importance for interventional and vascular radiology,” Michael A. Choma, MD, PhD, principal investigator at the Yale Biophotonics Laboratory in New Haven, Conn said.
“Biophotonics opens up the possibility to understand and manipulate things at the micrometer or even nanometer level. Micromanipulation techniques for example will allow to sort, move or modify cells. This links biophotonics to areas such as gene technology, stem cell research, tissue engineering, neuroscience or systems biology. Some of these techniques might also possibly allow for new ways of enhancing human performance features,” write Johann S. Ach; Beate Lüttenberg, Centre for Bioethics, University Münster
Biophotonic Applications
- The development of new classes of photonic probes and contrast agents to label structures and push the envelope of optical sensing to the limits of detection, resolution, and identification
- New imaging modalities and image/data fusion between optical imaging, spectroscopic techniques, and conventional medical imaging
- New optical approaches for noninvasive diagnosis, localization, and treatment of small tumors (i.e. either entirely new methods or major removal of limitations within existing technology)
- Development of biocompatible detection technologies that could serve as massively parallel interfaces for communicating with cells and tissue such as neural tissue
- Novel methods for “endoscopic” optical imaging at the subcellular level
- Noninvasive optical sensing techniques to detect key physiological and molecular concentrations invivo for anemia, jaundice, dehydration, glucose levels, drug levels, etc.
- Innovative methods for fluorescent labeling of macromolecules, use of enzyme ctivated fluorophores, new compositions of matter/methods of fabrication of multicolor probes for invo diagnostics such as marking and detection of tumors
Affordable light-based technologies also play a key role in meeting global healthcare challenges. As an example, Brian Wilson from the University of Toronto in Canada, described his research team’s development of a mobile phone-based fluorescence imager that detects bacteria in infected wounds and guides the clinician to areas requiring decontamination prior to bandaging. “A clinical trial showed that this makes a significant difference in terms of outcome, and it is now being commercialized,” he noted.
Thierry Livache from the CREAB group at CEA Grenoble (France) has developed a biochip that uses surface plasmon resonances (SPR) for molecular interaction measurement on microsystems. The chips provide a number of different sensors on the surface. Once in contact with the right molecule, they show SPR which can be electronically imaged. This technique may help to track different bacteria species on surfaces or to follow salmonella in large food samples
Biophotonics technology
Advancements in a number of fields, including lasers, camera and imaging technology, advanced neural networks have shifted the center of technology innovation. Many companies have advancements in technology related to biophotonics.
The targets of interest occur in a complex environment, in which signal specificity and sensitivity are challenged to overcome background noise. These sensitivity limitations are being addressed in several ways. Detectors and detection systems are becoming more sensitive, enabling better signal collection with lower noise. The uniqueness of signal against biological background, separation of the signal from the noise, and data processing are also employed. Imaging speed is rapidly advancing, such as sweptsource OCT systems with MHz axial scan rates, requiring also the corresponding use of faster detectors.
Biophotonic imaging will reap enormous benefits from recent advances in machine- and deep-learning algorithms. Coupled with the computational power that is now available and the immense information content of images, deep-learning can reveal patterns in images and will couple with computer-aided-diagnosis to reveal patterns that are
otherwise not visualized.
Through the use of synthetic biology and discovery chemistry, brighter fluorescent and luminescent proteins, enzymes, and chemical probes are being created. The convergence of biophotonics with nanosciences and nanotechnologies is rapidly expanding the ability for highly multiplexed detection/imaging as, for example, in surface-enhanced Raman scattering (SERS) nanoparticles as well as periodic array-based substrates for SERS and surface-enhanced spectroscopies in general.
Nanotechnology has helped in the improvement of the sensing phenomenon by the use of nanomaterials ranging from nanocantilevers, nanowires, nanoparticles, nanorods, and nanotubes. Nanomaterials such as carbon nanotubes and indium oxide nanowires are widely used for the construction of nanobiosensors. The most promising nanobiosensors technology is said to be based on the electronic detection of the target molecule such as Field Effect Transistor (FET) nanosensor.
The role of fluorescence in COVID-19 testing
For diagnosing active infections of COVID-19, the primary testing protocols involve using a process known as reverse-transcription polymerase chain reaction (rt-PCR). In traditional PCR, a strand of DNA is denatured, causing it to “unzip” and split into two halves. Next, a polymerase is added along with a DNA-coded primer designed for the genome of interest. The polymerase initializes a chemical process by which the two halves of the DNA strain are each “filled in” with the appropriate nucleic acid, creating two complete DNA molecules. This process is repeated, creating four molecules, then eight, then 16, and so on, exponentially increasing the total amount of DNA.
This amplification only takes place if the base DNA sequence matches that of the primer, making it a highly selective measurement technique to confirm the presence of a particular antigen, like SARS-CoV-2, the virus that causes COVID-19. It should be noted that since coronavirus is a single-stranded RNA virus, additional steps must be taken first to convert the RNA to DNA hence the need for reverse transcription, but the result is still the same.
A small RNA probe with a fluorophore linked to one end and a quenching molecule attached to the other end is used to tag the target DNA. The presence of the quenching molecule deactivates the fluorophore when it is nearby, rendering it inert in solution, but after bonding with the target RNA, the polymerase will cause the fluorophore to be released and activated . As the PCR process repeats, the fluorescence signal will eventually become strong enough to be detected. Therefore, every PCR testing system requires an excitation source (typically an LED), a photodetector, and multiple optical filters.
Antibody detection is also a powerful tool for determining if someone was previously infected and tracking vaccination efficacy. Currently, enzyme-linked immunosorbent assay (ELISA) is the gold standard for antibody testing. An ELISA assay consists of an antigen immobilized via an antibody bound to a substrate, typically located in the well of a microplate array. When exposed to the sample, if antibodies are present in the serum, they will also bind to the antigen. A secondary antibody conjugated to a reporter molecule is then added to the well to allow for detection using either colorimetry or fluorescence. Therefore, ELISA testing systems require similar optical components as in rt-PCR testing systems. As a result, every rt-PCR antigen test and ELISA antibody test for COVID-19 are not just tangentially dependent on photonics, but instead, at its core, fundamentally an optical analysis.
Developing point-of-care rapid screening
The value of ELISA and PCR testing cannot be understated, but unfortunately, neither method is well suited to rapid point-of-care (POC) testing. For example, even though ELISA is typically performed in a 96 well plate, allowing a large number of samples to be tested simultaneously, the total measurement time can still take several hours due to sample incubation times. Modern spectroscopic techniques have been opening up pathways to POC medical diagnostics. Two of the most promising POC screening methods are surface plasmon resonance (SPR) sensors and surface-enhanced Raman spectroscopy (SERS) immunoassays.
In April 2020, a research team led by Jean-François Masson, Chemistry Professor at the University of Montreal and CTO at Affinité Instruments (both in Montreal, QC, Canada) successfully demonstrated SPR as a means of quantitatively determining anti-SARS-CoV-2 antibodies in undiluted serum using portable SPR. Masson and his collaborators used a P4-SPR, a mobile SPR sensor from Affinité Instruments, for their analysis. The P4-SPR is designed to analyze crude biofluids such as blood plasma or blood serum, which can be injected directly into the instrument without pre-processing. This greatly simplifies the testing process and makes it far more intuitive to field technicians. Additionally, since the unit is only 175 × 155 × 55 mm in dimension and weighs less than 1.3 kg, the portability combined with the ability to measure blood serum directly makes portable SPR ideal for POC antibody testing.
POC antibody detection with SPR sensors
Surface plasmons are surface waves generated by coherent oscillation of conduction band electrons along the interface between a metal and a dielectric. As a result, surface plasmons are extremely sensitive to changes in a material’s dielectric constant, which is equal to the square root of the index of refraction. SPR sensors can, therefore, rapidly measure the changes in dielectric constant by refractometry, interferometry, or spectroscopy.
SPR sensors are constructed by depositing a thin noble metal film (such as gold, silver, or copper) on a glass substrate, and then an antigen can be bound to the metallic surface using a similar biochemical structure as described for ELISA. As antibodies attach to the antigens on the sensor’s surface, the dielectric constant slightly changes, resulting in a change to the resonant frequency of the surface plasmons. Therefore, SPR can provide rapid label-free detection since there is no need for secondary conjugation.
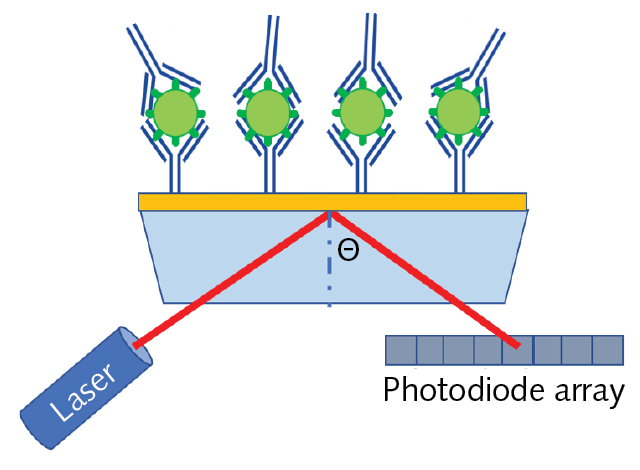
POC antigen detection with SERS assays
Surface-enhanced Raman spectroscopy (SERS), which is an enhancement technique based on the coupling of local surface plasmon resonance (LSPR) and Raman scattering, is now one of the leading candidates being investigated as a possible solution for rapid COVID-19 screening. LSPR relies on nanostructured noble metals to locally increase the surface charge density to amplify the intensity of the surface plasmons, enhancing the intensity of the Raman scatter by up to a factor of 106. This extreme sensitivity, combined with Raman spectroscopy’s innate specificity, has made SERS a leading candidate for rapid POC COVID-19 screening.
Photonic technology enables fast, sensitive saliva test for COVID-19
Researchers from ICFO-The Institute of Photonic Sciences and IrsiCaixa AIDS Research Institute, both in Spain, have demonstrated a new low-cost portable instrument that manipulates light and fluid for fast and reliable detection of SARS-CoV-2 in saliva samples.
The new instrument, which the researchers call a flow virometry reader, is based on a modification of flow cytometry, a laser-based technique that uses fluorescence to count or analyze cells. Rather than counting cells, the new device detects light emission from fluorescent antibodies that bind to specific viral particles. It can offer quantitative results in less than 30 minutes using an instrument that is about the size of a shoebox.
To run a test, a saliva sample is introduced in a solution that contains fluorescent antibodies that will attach to SARS-CoV-2 particles. The sample is then processed by the flow virometry reader, which uses a single microfluidic channel to pass the sample through a laser illumination and detection setup. If viral particles are present in the solution, they change the sample’s fluorescence in a detectable way, providing information that can be used to calculate the viral concentration.
In the Optica Publishing Group journal Biomedical Optics Express, the researchers report that the system correctly detected 91.2% of COVID-19 positive cases and 90% of negative cases in tests of more than 50 previously frozen saliva samples. The test can measure very small quantities of virus like PCR tests but is as fast as rapid antigen tests.
“Due to its low cost and simple design, the flow virometry reader could be particularly useful for low-income countries with limited access to healthcare,” said Ongaro. Also, non-specialist users can easily operate the device, and testing can be carried out anywhere since no specialized laboratory is required, explained Marisa Rodriguez and Jorge Carrillo, researchers from IrsiCaixa AIDS Research Institute.
The researchers are now working to develop an integrated flow virometry reader for simultaneous SARS-CoV-2 antigen and antibody analysis. Such a testing device could identify active infections as well as provide information on the immune response to a previous infection or vaccination. This will allow fast checking of vaccine efficiency and help to determine whether additional vaccination boosters are needed.
Mobile Device to Provide Instant Diagnosis of Heart Disease
EU-funded CARDIS project that has developed a prototype medical device for the diagnosis of various CVDs such as arterial stenosis and heart failure. Its technology is based on silicon photonics. The press release states: “The operating principle of the device is Laser Doppler Vibrometry (LDV), in which a very low-power laser is directed towards the skin overlying an artery. The skin’s vibration amplitude and frequency, resulting from the heart beat, are extracted from the Doppler shift of the reflected beam.” It adds that the device can scan “multiple points on the skin above the artery in parallel. At the heart of the system is a silicon photonics chip containing the optical functionality of the multi-beam LDV device.”
If the results show that the technology can detect CVDs at an early stage, the project will start high-volume production. “One of the benefits of the silicon photonics technology is that at high volumes, the chip can be produced at low cost,” explains imec. The CARDIS (Early stage CARdio Vascular Disease Detection with Integrated Silicon Photonics) project was set up to design a mobile robust and low-cost device for the screening of arterial stiffness and detection of stenosis and heart failure.
A press release by project partner Interuniversitair Micro-Electronica Centrum (imec) notes that the instrument requires minimal physical contact with the patient and minimal user skills for the screening of arterial stiffness. This condition involves alterations in the mechanical properties of arteries. Much effort has focused on how best to measure this. Its assessment “by measurement of aortic pulse wave velocity (aPWV) is included in the latest guidelines for CVD risk prediction and it is a key marker for hypertension” as explained in the press release. However with currently available tools it’s difficult to screen a large number of patients for this condition at a typical general practitioner’s office.
Biophotonics Market worth 50.18 Billion USD by 2020
The global biophotonics market is expected to grow from $41.76 billion in 2020 to $45.67 billion in 2021 at a compound annual growth rate (CAGR) of 9.4%. The market is expected to reach $63.04 billion in 2025 at a CAGR of 8%. The growth is mainly due to the companies resuming their operations and adapting to the new normal while recovering from the COVID-19 impact, which had earlier led to restrictive containment measures involving social distancing, remote working, and the closure of commercial activities that resulted in operational challenges. The entire supply chain was disrupted, impacting the market negatively.
The increasing demand for minimally invasive surgeries is contributing to the growth of the biophotonics market. Inside imaging (Endoscopy) is an instrument used by doctors to work on the internal organs of the body. Surgeons, using endoscopy can view internal problems without making a large incision.
Optical engineering and imaging technologies are playing a vital role in the evolving field of minimally invasive surgeries by enabling to visualize the manipulation of tissue at remote internal sites. According to the plastic surgery statistics report 2019, the minimally invasive cosmetic procedures reached 16.3 million in 2019, an increase of 2% over the previous year. Therefore, the surge in the number of minimally invasive surgeries annually worldwide is generating higher revenues for the biophotonics market.
The biophotonics market consists of revenues generated from the sales of biophotonic instruments combining optics, nanotechnology, photonics, and biotechnology. Biophotonics is the combination of photonics and biology and is a multidisciplinary research field embracing all light-based technologies applied to life sciences and medicine. It refers to the use of photonic or optical means to examine, control, and track biological processes at various levels of biology: cellular, tissue, molecular, and organism level.
High prices of biophotonic-based devices or instruments are anticipated to limit the growth of the biophotonics market. The price of biophotonics is comparatively higher than that of conventional instruments. They are considered as more complex attributing to the integration of biological units and the generation, manipulation, and detection of light units, thereby rising their price.
Market Segments
The biophotonics market is segmented by product technology into in-vitro, in-vivo. It is also segmented by application into see-through imaging, inside imaging, spectro molecular, surface imaging, microscopy, light therapy, biosensors, others and by end-use into diagnostics, therapeutic, tests, others.
The largest segment for biophotonics market is see-through imaging, it accounted for a market share of about 40% in 2014. There have been significant developments in the field of see-through imaging techniques in recent years, especially in drug discovery and medical diagnostics. Over the years, see-through imaging has emerged as an effective tool for in-vitro and in-vivo imaging, and become an integral part of biomedical research. These techniques have helped in enhancing the knowledge of disease detection and progression, thereby expediting the development of effective therapeutics.
See through imaging are of three kinds: x-ray imaging, optical molecular imaging and photoacoustic imaging. Spectromolecular imaging is used to measure a given molecule at its qualitative and quantitative level. Some of the spectromolecular imaging techniques commonly applied are Infrared spectroscopy, UV-visible spectroscopy, Raman spectroscopy and colorimetric systems. Microscopy (optical microscopy) is used commonly for in-vitro applications.
There are three types of microscopy, optical, electron and scanning probe microscopy. Surface imaging is a technique used to understand the physical or molecular makeup of an in-vivo biomolecule. Light therapy (phototherapy) involves exposing the body to different wavelengths of light. It is a technique used to treat seasonal affective disorders (SAD) like depression. The biosensor is an analytical device that consists of a biological component (enzyme, antibody, nucleic acid) and a transducer. Analytical sensing technique has been used for analytical instruments as a detector unit/device.
The biophotonic sensor market has tremendous growth opportunity over the forecast period due to the growing application of these sensors in the military and medical sectors. Fiber-optic sensors are being deployed in new applications such as consumer electronics and biomedical sensing. Hospitals across the globe are adopting advanced medical equipment such as biomedical sensors, which deploy fiber-optic sensors, to improve diagnosis, monitoring, and treatment of patients. This will support the demand for fiber-optic sensors during the forecast period, according to market research study released by Technavio.
In the medical sector, they help in the development of drugs and vaccines as well as in therapeutics and diagnostics. The ability of these sensors to offer quick and precise analysis has increased their use in the medical sector, especially in microfluidic devices. The need of biophotonics systems for the detection of biochemical agents will boost the demand of the biophotonics market in the defense sector.
Biophotonics systems are also being developed for the use in environmental monitoring. Since environmental concerns such as increasing pollution and global warming are common issues faced by various nations, the use of biophotonics for environment monitoring is expected to grow in the coming years.
The key companies in this market include Affymetrix, Inc. (U.S.), Andor Technology Ltd. (U.K.), Becton, Dickinson and Company (U.S.), Carl Zeiss AG (Germany), FEI Company (U.S.), Hamamatsu Photonics K.K. (Japan), Lumenis Ltd. (Israel), Olympus Corporation (Japan), PerkinElmer, Inc. (U.S.), and Zecotek Photonics Inc. (Canada). Other companies are Thermo Fisher Scientific, OPGEN, NU Skin Enterprises, PG Photonics Corp, Idex Corp, Toshiba, Procter & Gamble, Horiba, Precision Photonics Corp, Roche Group, GE, Philips, Affymetrix, Inc., Andor Technology Ltd., Zenalux Biomedical Inc., Glenbrook Technologies Inc., and Oxford Instruments Plc
European partnership for thrust on Biophotonics
The field of life sciences is heavily dependent on bulky and expensive optical systems and would benefit enormously from low cost photonic implementations. However this field requires a visible light PIC-technology. Proof of concept demonstrations are abundant, but pilot line and manufacturing capacity is limited, inhibiting industrial take up.
PIX4life will drive the future European RTD in visible photonic applications for life sciences by bridging technological research (via participation of 2 academic and 2 research institutes) towards industrial development (via participation of a foundry, two large companies and 9 fabless SME’s, either technology suppliers or life science end users).
PIX4Life is a pilot for a state-of-the-art photonics platform for health applications. Recently, a promising Silicon Nitride Photonic Integrated Circuit technology was researched to make compact, low cost detection and imaging systems in the visible range and the TriPleXTM and BioPIX platforms were developed. PIX4Life will scale up these platforms in order to bring Silicon Nitride based systems towards commercial production and industrial take up. This will open a multibillion market of products including biosensors, cytometers, DNA sequencers, gas sensors, microscopes, medical imagers and more. The PIX4Life pilot line will drive European leadership in health applications by making this technology accessible to industrial and academic customers together with the necessary design, packaging and test services. The project brings together 15 leading organisations from 7 European countries and is coordinated by IMEC, Belgium.
MIRPHAB (http://www.mirphab.eu/) is a pilot line for prototyping and production of innovative sources and sensors in the Mid-IR range, for the detection of chemicals in gas and liquids. MIRPHAB platform is based on miniaturized laser systems and will allow the manufacturing of compact, low cost and low power consumption sensing devices which can be used for safety, security and environmental applications. The industry partners involved in MIRPHAB are committed to deploy new products swiftly in the market and achieve prompt take up in the environmental and chemical sensing areas.The project brings together 18 leading organisations from 9 European countries and is coordinated by CEA-Leti, France.
References and Resources also include:
https://www.sciencedaily.com/releases/2020/09/200922172525.htm
 International Defense Security & Technology Your trusted Source for News, Research and Analysis
International Defense Security & Technology Your trusted Source for News, Research and Analysis
