The rapid increase in global energy consumption and the environmental impact of traditional energy resources pose serious challenges to human health, energy security, and the environment; and reveal a growing need to develop new types of clean and sustainable energy solutions such as electric vehicles with low exhaust emissions. However the main factors discouraging motorists in Germany from switching to electric vehicles are the high investments cost, their short driving ranges and the lack of charging stations. Another major obstacle en route to the mass acceptance of electric cars is the charging time involved. The minutes involved in refueling conventional cars are so many folds shorter that it makes the situation almost incomparable.
Conventional electrochemical energy storage systems, including lithium-ion batteries (LIBs), have a high voltage range and energy density, but are subject to safety issues raised by flammable organic electrolytes, which are used to ensure the beneficial properties. Additionally, they suffer from slow electrochemical reaction rates, which lead to a poor charging rate and low power density with a capacity that fades quickly, resulting in a short cycle life.
However, the charging durations could be dramatically shortened with the inclusion of supercapacitors, also known as ultracapacitor or double-layer capacitor. Supercapcitors differs from a regular capacitor in that it has very high capacitance. Supercapacitors combine the high energy-storage-capability of conventional batteries with the high power-delivery-capability of conventional capacitors.
A capacitor stores energy by means of a static charge as opposed to an electrochemical reaction. It has two electrodes dipped in an electrolyte and separated by a thin insulator. Charging is done by applying a voltage differential on the positive and negative plates of the capacitor. When the electrodes are charged, an electric field is created between them, which allows energy to be stored. This is similar to the buildup of electrical charge when walking on a carpet. Touching an object releases the energy through the finger.
There are three types of capacitors and the most basic is the electrostatic capacitor with a dry separator. This classic capacitor has very low capacitance and is mainly used to tune radio frequencies and filtering. The size ranges from a few pico-farads (pf) to low microfarad (μF). The electrolytic capacitor provides higher capacitance than the electrostatic capacitor and is rated in microfarads (μF), which is a million times larger than a pico-farad. These capacitors deploy a moist separator and are used for filtering, buffering and signal coupling. Similar to a battery, the electrostatic capacity has a positive and negative that must be observed.
The third type is the supercapacitor, rated in farads, which is thousands of times higher than the electrolytic capacitor. The supercapacitor is used for energy storage undergoing frequent charge and discharge cycles at high current and short duration. Supercapacitors have many advantages over the LI-ion battery, they charge extremely fast ( 1-10 secs) compared to 10-60 min of LI Ion. They have high specific power, stroing 10,000 watts per kg compared to 1000-3000 by LI ion and have millions of charge cycles compared to 500 of battery.
Supercapacitors are useful for releasing large bursts of energy quickly, in a camera flashlight, for example, or in dynamic brakes in cars, trains and elevators. They not only get charged quickly, but also last longer and are less toxic than batteries. These alternative energy storage devices are fast charging and can therefore better support the use of economical energy in electric cars.

Yet, despite their potential, supercapacitors, at present, have certain drawbacks that are preventing their widespread use. One major issue is that they have low energy density; that is, they store insufficient energy per unit area of their space. Their specific energy is very small 5 Wh/ Kg compared to 100-200 of battery. Another growing issue in supercapacitor production—mainly for smartphone and electric car technologies—is sustainability.
Supercapacitors are devices that could one day replace batteries used in electric cars, cell phones or laptops, because they charge very quickly, and work at almost 100 percent efficiency. But they are usually bulky and can only store limited amounts of energy. Reducing their size without losing efficiency has proved challenging.
To improve the performance of state-of-the-art supercapacitors to meet the stringent requirements for the applications like hybrid electric vehicles (HEVs) to industrial electric utilities, portable, transparent and wearable electronics, new electrode materials with superior properties over those of current activated carbon electrodes are needed and new device structures are highly desirable. Fabricating them using existing methods is also costly and complicated.
Supercapacitors
Key requirements for supercapacitor electrodes are a large surface area and conductivity, combined with a simple production method. The larger the surface area of the electrodes, the greater is the charge that can be stored.
Several types of electrodes have been tried and the most common systems today are built on the electrochemical double-layer capacitor that is carbon-based, has an organic electrolyte and is easy to manufacture. Activated carbons are used for the electrodes in capacitors, but these are limited by low voltage in single cells, the building blocks that make up capacitors. This means that a large number of cells must be stacked together to achieve the required voltage.
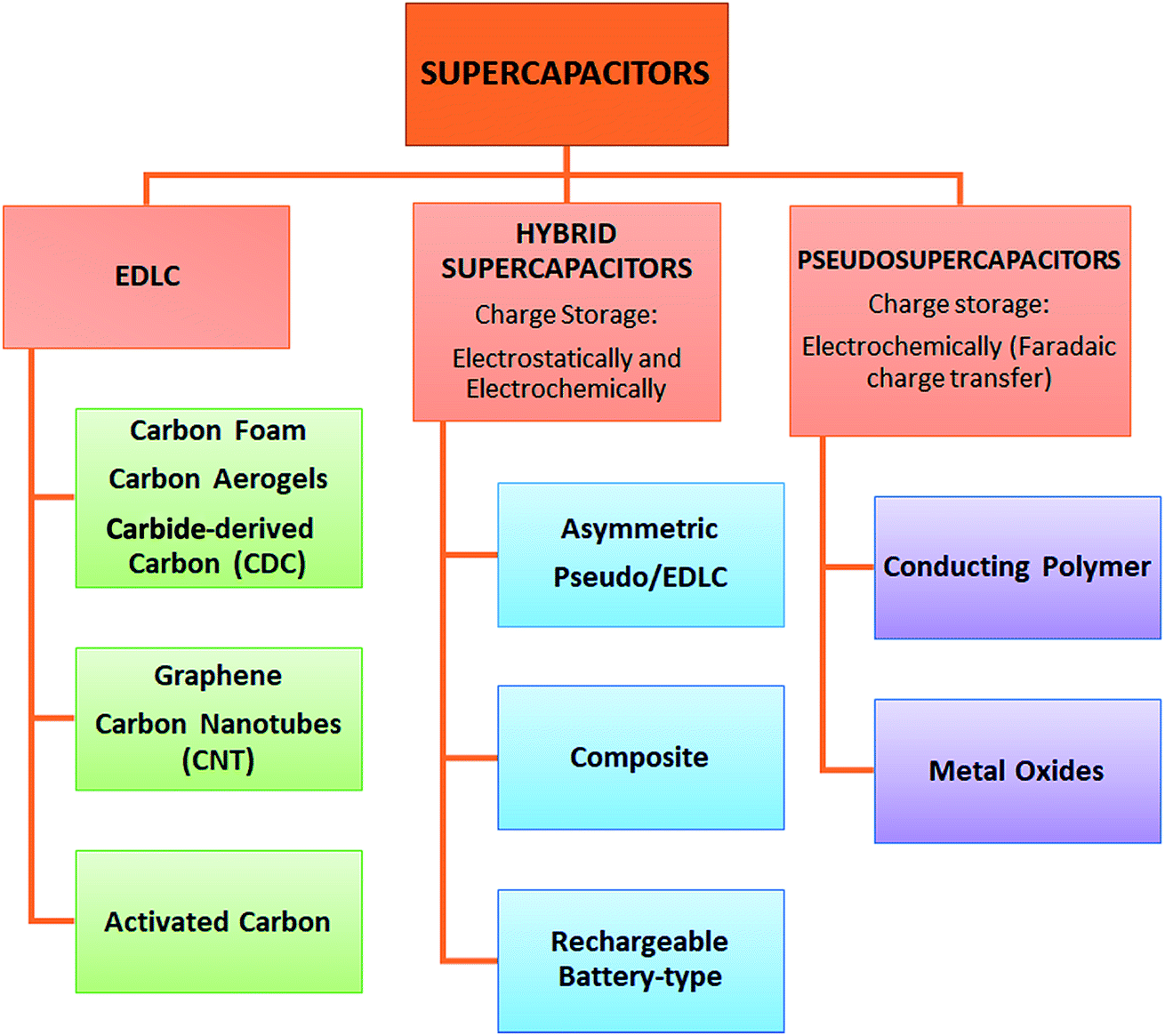
Electrostatic double-layer capacitors (EDLCs) use carbon electrodes or derivatives with much higher electrostatic double-layer capacitance than electrochemical pseudocapacitance, achieving separation of charge in a Helmholtz double layer at the interface between the surface of a conductive electrode and an electrolyte. The separation of charge is of the order of a few ångströms (0.3–0.8 nm), much smaller than in a conventional capacitor.
Electrochemical pseudocapacitors use metal oxide or conducting polymer electrodes with a high amount of electrochemical pseudocapacitance additional to the double-layer capacitance. Pseudocapacitance is achieved by Faradaic electron charge-transfer with redox reactions, intercalation or electrosorption.
Hybrid capacitors, such as the lithium-ion capacitor, use electrodes with differing characteristics: one exhibiting mostly electrostatic capacitance and the other mostly electrochemical capacitance.
Nanotechnology enhanced Supercapacitors
Nanotechnology has opened up new frontiers by offering unique enabling technologies and new materials for energy storage. Recently, carbon nanomaterials (especially, carbon nanotubes and graphene) have been widely investigated as effective electrodes in supercapacitors due to their high specific surface area, excellent electrical and mechanical properties.
In particular, graphitic carbon nanomaterials (e.g. carbon nanotubes, graphene sheets) have been playing a more and more important role in the development of high-performance supercapacitors. Owing to their large surface area, high mesoporosity and electrolyte accessibility, and good electrical properties, carbon nanomaterials, especially graphene and carbon nanotubes (CNTs), are very promising candidates to replace activated carbons as the electrode materials in high-performance supercapacitors
CNTs with a high aspect ratio, large specific surface area (SWNT >1600 m2 g−1, MWNT >430 m2 g−1) as well as good mechanical and electrical (∼5000 S cm−1) properties have been widely used as the active electrodes in supercapacitors. CNTs have higher electrical conductivity and large readily accessible surface areas. However, single-walled carbon nanotubes are very likely to stack in bundles, leading to only the outermost portion of the CNTs being available for ion accumulation, which results in a lower specific capacitance.
Recently, graphene-based electrodes for supercapacitor applications have been widely investigated due to the material’s high specific surface area, excellent electrical properties and its chemical and thermal stability. Graphene is an excellent electrode material, because of its properties like high mechanical flexibility, strength, extremely high specific surface area of up to 2,600 m2/g and high electrical conductivity. It consists of an ultrathin monolayer lattice made of carbon atoms. When used as an electrode material, it greatly increases the surface area with the same amount of material. From this aspect, graphene is showing its potential in replacing activated carbon – the material that has been used in commercial supercapacitors to date – which has a specific surface area between 1000 and 1800 m2/g.
In addition, graphene-based supercapacitors (G-SCs) are able to act as flexible and deformable energy storage devices in electronic skins, wearable displays, curved smartphones, etc.
Graphene electrodes significantly improve energy efficiency
Scientists have long sought high-performance materials for supercapacitors that can meet the requirements for energy-intensive applications such as cars. “It is very challenging to find materials that can both operate at high-voltage and remain stable under harsh conditions,” says Hirotomo Nishihara, materials scientist at Tohoku University and co-author of the paper.
Crucially, the new material has higher single-cell voltage, reducing the stacking number and allowing devices to be more compact. Nishihara and his colleagues collaborated with the supercapacitor production company TOC Capacitor Co. to develop a new material that exhibits extraordinarily high stability under conditions of high voltage and high temperature.
The new material is a sheet made from a continuous three-dimensional framework of graphene mesosponge, a carbon-based material containing nanoscale pores. A key feature of the materials is that it is seamless—it contains a very small number of carbon edges, the sites where corrosion reactions originate, and this makes it extremely stable.
They also tested commercial graphene-based materials, including single-walled carbon nanotubes, reduced graphene oxides, and 3-D graphene, using activated carbons as a benchmark for comparison. They showed that the material had excellent stability at high temperatures of 60 °C and high voltage of 3.5 volts in a conventional organic electrolyte. Significantly, it showed ultra-high stability at 25°C and 4.4 volts—2.7 times higher than conventional activated carbons and other graphene-based materials. “This is a world record for voltage stability of carbon materials in a symmetric supercapacitor,” says Nishihara. The new material paves the way for development of highly durable, high-voltage supercapacitors that could be used for many applications, including motor vehicles.
Nanoclusters on graphene make fast and powerful aqueous hybrid capacitor
A KAIST research team made it one step closer to realizing safe energy storage with high energy density, high power density, and a longer cycle life (Advanced Functional Materials, “Synthesis of Pseudocapacitive Porous Metal Oxide Nanoclusters Anchored on Graphene for Aqueous Energy Storage Devices with High Energy Density and Long Cycling Stability along with Ultrafast Charging Capability”). This hybrid storage alternative shows power density 100 times faster than conventional batteries, allowing it to be charged within a few seconds. Hence, it is suitable for small portable electronic devices.
Capacitors based on aqueous electrolytes are receiving a great deal of attention because they are considered to be safe and environmentally friendly alternatives. However, aqueous electrolytes lag behind energy storage systems based on organic electrolytes in terms of energy density due to their limited voltage range and low capacitance.
Here, Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability and his team developed an aqueous hybrid capacitor (AHC) that boasts high energy density, high power, and excellent cycle stability by synthesizing two types of porous metal oxide nanoclusters on graphene to create positive and negative electrodes for AHCs.
The porous metal oxide nanoparticles are composed of nanoclusters as small as two to three nanometers and have mesopores that are smaller than five nanometers. In these porous structures, ions can be rapidly transferred to the material surfaces and a large number of ions can be stored inside the metal oxide particles very quickly due to their small particle size and large surface area.
The team applied porous manganese oxide on graphene for positive electrodes and porous iron oxide on graphene for negative electrodes to design an aqueous hybrid capacitor that can operate at an extended voltage range of 2V. Professor Kang said, “This newly developed AHC with high capacity and power density driven from porous metal oxide electrodes will contribute to commercializing a new type of energy storage system. This technology allows ultra-fast charging within several seconds, making it suitable as a power source for mobile devices or electric vehicles where solar energy is directly stored as electricity.”
Sustainable graphene hybrid material provides high performance reported in Jan 2021
The team working with TUM chemist Roland Fischer has now developed a novel, powerful as well as sustainable graphene hybrid material for supercapacitors. It serves as the positive electrode in the energy storage device. The researchers are combining it with a proven negative electrode based on titan and carbon.
The new energy storage device does not only attain an energy density of up to 73 Wh/kg, which is roughly equivalent to the energy density of a nickel metal hydride battery, but also performs much better than most other supercapacitors at a power density of 16 kW/kg. The secret of the new supercapacitor is the combination of different materials – hence, chemists refer to the supercapacitor as “asymmetrical.”
As a basis, they used the novel positive electrode of the storage unit with chemically modified graphene and combined it with a nano-structured metal organic framework, a so-called MOF. Decisive for the performance of graphene hybrids are on the one hand a large specific surface and controllable pore sizes and on the other hand a high electrical conductivity. “The high performance capabilities of the material is based on the combination of the microporous MOFs with the conductive graphene acid,” explains first author Jayaramulu Kolleboyina, a former guest scientist working with Roland Fischer.
A large surface is important for good supercapacitors. It allows for the collection of a respectively large number of charge carriers within the material – this is the basic principle for the storage of electrical energy. Through skillful material design, the researchers achieved the feat of linking the graphene acid with the MOFs. The resulting hybrid MOFs have a very large inner surface of up to 900 square meters per gram and are highly performant as positive electrodes in a supercapacitor.
However, that is not the only advantage of the new material. To achieve a chemically stable hybrid, one needs strong chemical bonds between the components. The bonds are apparently the same as those between amino acids in proteins, according to Fischer: “In fact, we have connected the graphene acid with a MOF-amino acid, which creates a type of peptide bond.”
The stable connection between the nano-structured components has huge advantages in terms of long term stability: The more stable the bonds, the more charging and discharging cycles are possible without significant performance impairment. For comparison: A classic lithium accumulator has a useful life of around 5,000 cycles. The new cell developed by the TUM researchers retains close to 90 percent capacity even after 10,000 cycles.
Manganese oxide based Supercapacitor Promises Storage, High Power And Fast Charging
A new supercapacitor based on manganese oxide could combine the storage capacity of batteries with the high power and fast charging of other supercapacitors, according to researchers at Penn State and two universities in China. “Manganese oxide is definitely a promising material,” said Huanyu “Larry” Cheng, assistant professor of engineering science and mechanics and faculty member in the Materials Research Institute, Penn State. “By combining with cobalt manganese oxide, it forms a heterostructure in which we are able to tune the interfacial properties.”
The group started with simulations to see how manganese oxide’s properties change when coupled with other materials. When they coupled it to a semiconductor, they found it made a conductive interface with a low resistance to electron and ion transport. This will be important because otherwise the material would be slow to charge. “Exploring manganese oxide with cobalt manganese oxide as a positive electrode and a form of graphene oxide as a negative electrode yields an asymmetric supercapacitor with high energy density, remarkable power density and excellent cycling stability,” according to Cheng Zhang, who was a visiting scholar in Cheng’s group and is the lead author on a paper published recently in Electrochimica Acta.
The group has compared their supercapacitor to others and theirs has much higher energy density and power. They believe that by scaling up the lateral dimensions and thickness, their material has the potential to be used in electric vehicles. So far, they have not tried to scale it up. Instead, their next step will be to tune the interface where the semiconducting and conducting layers meet for even better performance. They want to add the supercapacitor to already developed flexible, wearable electronics and sensors as an energy supply for those devices or directly as self-powered sensors. The paper is “Efficient Coupling of Semiconductors into Metallic MnO2@CoMn2O4 Heterostructured Electrode with Boosted Charge Transfer for High-performance Supercapacitors.” The National Natural Science Foundation of China and the Science Research Fund of Guizhou Province, China supported this research.
Boron-doped nanodiamond, as electrode in the supercapacitors
Scientists first attempted to solve this problem by using organic solvents as the electrolyte–the conducting medium–inside supercapacitors to raise the generated voltage (note that the square of the voltage is directly proportional to energy density in energy storage devices). But organic solvents are costly and have low conductivity. So, perhaps, an aqueous electrolyte would be better, the scientists thought. Thus, the development of supercapacitor components that would be effective with aqueous electrolytes became a central research topic in the field.
In the aforementioned recent study, published in Scientific Reports, Dr Kondo and group from the Tokyo University of Science and Daicel Corporation in Japan explored the possibility of using a novel material, the boron-doped nanodiamond, as electrode in the supercapacitors–electrodes are the conducting materials in a battery or capacitor that connect the electrolyte with external wires, to transport current out of the system. This research group’s choice of electrode material was based on the knowledge that boron-doped diamonds have a wide potential window, a feature that enables a high-energy storage device to remain stable over time. “We thought that water-based supercapacitors producing a large voltage could be realized if conductive diamond is used as an electrode material,” Dr Kondo says.
The scientists used a technique called the microwave plasma-assisted chemical vapor deposition, MPCVD, to manufacture these electrodes and examined their performance by testing their properties. They found that in a basic two-electrode system with an aqueous sulfuric acid electrolyte, these electrodes produced a much higher voltage than did conventional cells, resulting in much higher energy and power densities for the supercapacitor. Further, they saw that even after 10,000 cycles of charging and discharging, the electrode remained very stable. The boron-doped nanodiamond had proven its worth.
Thus, as Dr Kondo has said, “the boron-doped nanodiamond electrodes are useful for aqueous supercapacitors, which function as high-energy storage devices suitable for high-speed charging and discharging.”
Sustainable highly conductive electrode materials from ultrathin carbon nanofiber aerogels
Carbon aerogels are ultralight, conductive materials, which are extensively investigated for applications in supercapacitor electrodes in electrical cars and cell phones.
The authors also demonstrated that their wood-derived carbon aerogel worked well as a binder-free electrode for supercapacitor applications. The material displayed electrochemical properties comparable to commercial electrodes.
Another growing issue in supercapacitor production—mainly for smartphone and electric car technologies—is sustainability. However, sustainable and economical production of carbon aerogels as supercapacitor electrode materials is possible, propose Shu-Hong Yu and colleagues from the University of Science and Technology of China, Hefei, China.
Chinese scientists have now found a way to make these electrodes sustainably. The aerogels can be obtained directly from cellulose nanofibrils, the abundant cell-wall material in wood, finds the study reported in the journal Angewandte Chemie.
Washington University Researchers develop shatterproof Supercapacitors
Researchers at Washington University in St. Louis made an energy storage device that can withstand a hammer striking it more than 40 times. The shatterproof supercapacitor is also nonflammable, unlike lithium-ion batteries. The new work is the cover story of the April 2019 issue of the journal Sustainable Energy and Fuels.
“Accidentally dropping electronics, such as a laptop or cellphone, is a common scenario that may lead to the failure of the device,” said Julio D’Arcy, assistant professor of chemistry in Arts & Sciences. “In some cases, energy storage devices catch on fire due to impact-caused failure. The chance of impact damage will only increase as electronics become more flexible and worn on the human body.”
Hongmin Wang, a PhD candidate in chemistry who works in D’Arcy’s lab, led the effort to create the new material. By controlling the formation of rust in solution, researchers grew a micrometer-thick porous mat of conducting fibers affixed to a soft, pliable layer of organic plastic. The result is somewhat similar to an open-faced sandwich.
“This is the same mechanism that is responsible for the formation of rust on the surface of a wet piece of steel,” D’Arcy said. “Here, we have carefully designed the nanostructure orientation so that a polymer film assembles parallel to a rusted surface. It produces an interwoven mat of polymer nanofibers with a textile-like structure that is flexible and ideal for storing energy in a supercapacitor.”
The researchers bent their new material to different angles over and over again. They hammered it repeatedly, and they also tested it against an impact equivalent to a car collision at 30 mph. The same amount of impact would fracture other materials such as metal and carbon. The device held up well against these extreme tests: after the first hammer strike, it retained 80 percent of its ability to store energy at peak efficiencies; after 40 repeated strikes, it was still at 74 percent.
Production Methods
Initially, graphene was prepared by mechanical exfoliation. This kind of graphene shows ultrahigh conductivity; however, graphene prepared by mechanical exfoliation suffers from small size and irregular shape, which is not suitable for fabricating SC electrodes. In recent years, technological advances have been made in large-area graphene film preparation, such as reduction of graphene oxide (RGO) and chemical vapor deposition growth graphene (CVDG).
Therefore, tremendous preparation methods involving chemicals, high-temperature treatment, etc., have been investigated to fabricate G-SCs. In order to commercialise graphene-based supercapacitors, efficient large-scale production of appropriate quality graphene is crucial. Large-scale graphene sheets have been produced by chemical vapour deposition (CVD), ultrasonic exfoliation of graphene in solvents such as N-methylpyrrolidone (NMP), epitaxial growth on SiC, electrochemical exfoliation of graphite and solution-based chemical reduction of graphene oxide.
Although CVD and epitaxial growth can produce high quality graphene on substrates, both methods require high temperatures . Ultrasonic exfoliation of graphene in NMP can give high quality and uniform flakes, but the yield remains low (<50% 1-4 layers graphene) and the lateral flake dimensions are usually small (<1 μm). Electrochemical exfoliation of graphite is very simple, low-cost and less polluting in comparison to the other methods listed, and these features make it ideal for use in commercial graphene-based supercapacitors.
In addition, EEG has lower oxygen content and higher conductivity compared to graphene oxide and reduced graphene oxide. These factors make EEG a suitable candidate for high-power supercapacitor applications. Flexible EEG-based supercapacitors have been demonstrated with high rate capability and gravimetric capacitances ranging from 18.8 to 56.6 Fg−1, depending on the loading of EEG in the electrodes, which varied from 0.6 to 0.2 mg cm−2.
Recently, laser technologies have been adopted: laser reduction of graphene oxides (LRGOs), laser treatment of CVDGs (LCVDGs), and laser-induced graphene (LIG). Compared with the graphene prepared via mechanical exfoliation, thermal/chemical RGO and CVDG, laser-enabled graphene can be prepared in large-area films with high electrical conductivity, flexibility, and high chemical/physical stability. Importantly, compared with these fabricating methods, laser is a powerful tool to fabricate G-SCs, which avoids the need for additional toxic-reducing agents, high temperature, and inert gas protection. Also, laser technologies allow high resolution for miniaturized SC design and fabrication. Moreover, laser technologies permit programmable patterning in arbitrary shapes and are also compatible with other functional units for integration. Therefore, laser fabrication of G-SCs holds great potential for effective energy storage devices.
Indian Scientists develop Compact and flexible supercapacitor developed using simple spray coating method in 2018
Currently used supercapacitors cannot compete with batteries in energy storage; a supercapacitor with the same storage capacity as a regular battery would weigh up to 40 times as much. To make them both light and efficient, researchers have tried to use materials such as carbon nanotubes or reduced graphene oxide to prepare the electrodes. Using traditional lithography to fabricate them, however, creates bulk structures with less surface area for charges to move. The process is also expensive and time-consuming.
A lightweight, compact and efficient supercapacitor printed on a flexible plastic sheet has been developed by researchers at the Indian Institute of Science (IISc). In the current study, the IISc team created a compact supercapacitor by using a simple spray coating technique to deposit alternating layers of hybrid nanocomposites on a bendable plastic sheet. The layer-by-layer patterning increased the surface area and boosted the movement of charges, making the device more efficient than existing supercapacitors.
“We can actually print these supercapacitors anywhere, on any substrate; thus they can easily be mounted on any surface just like a simple spray on the walls,” says senior author Abha Misra, Associate Professor at the Department of Instrumentation and Applied Physics, IISc. The study was published in ACS Applied Materials and Interfaces.
Instead, Misra’s team used a simple spray technique to deposit thin, alternating layers of MnO2-coated carbon nanotubes (CNTs) and reduced graphene oxide (rGO). These layers were stacked on top of a stainless steel mask mounted on a standard PET plastic sheet. This type of patterning not only increased the surface area, but also positioned the materials strategically for charges to move efficiently.
The layered hybrid supercapacitor showed a much larger capacitance — a measure of how much energy could be stored — compared to structures that had only CNT, only rGO, or a random mix of the two materials. For the same size, it also showed greater storage capacity than existing supercapacitors reported to date. Bending the supercapacitor-printed sheet also did not affect its performance, making it useful for flexible energy storage applications.
Commercial Graphene based supercapacitors
Skeleton Technologies is Europe’s leading manufacturer of ultracapacitors, uses patented nanoporous carbide-derived carbon, or ‘curved graphene’ to deliver twice the energy density and 5 times the power density offered by other manufacturers. The ultracapacitors also ensure reliability by starting the vehicle in cold conditions or after prolonged periods in storage.
Huawei claims that its new charging technology that works with super-strong and super-conductive Graphene, ensures that batteries can be recharged 10 times faster without any expense of the battery lifetime.
At the battery symposium the Chinese smartphone manufacturer demonstrated the recharging of a 3000 mAh battery from 0 to 48% in five minutes. A 600mAh battery was recharged to 68% in just two minutes. For reference; the iPhone 6S has a 1750mAh battery and the Galaxy S6 has a 2550mAh battery.
“We are confident that this breakthrough in fast charging leads to a new revolution in electronic devices,” Huawei writes in a press release. “Soon we can recharge our batteries in the time it takes to pour a cup of coffee!” It goes without saying that superfast battery charging will greatly enhance the usage of electric bikes.
References and Resources also include:
https://www.nanowerk.com/nanotechnology-news2/newsid=51454.php
https://phys.org/news/2019-02-supercapacitor-material-energy-density-higher.html
 International Defense Security & Technology Your trusted Source for News, Research and Analysis
International Defense Security & Technology Your trusted Source for News, Research and Analysis