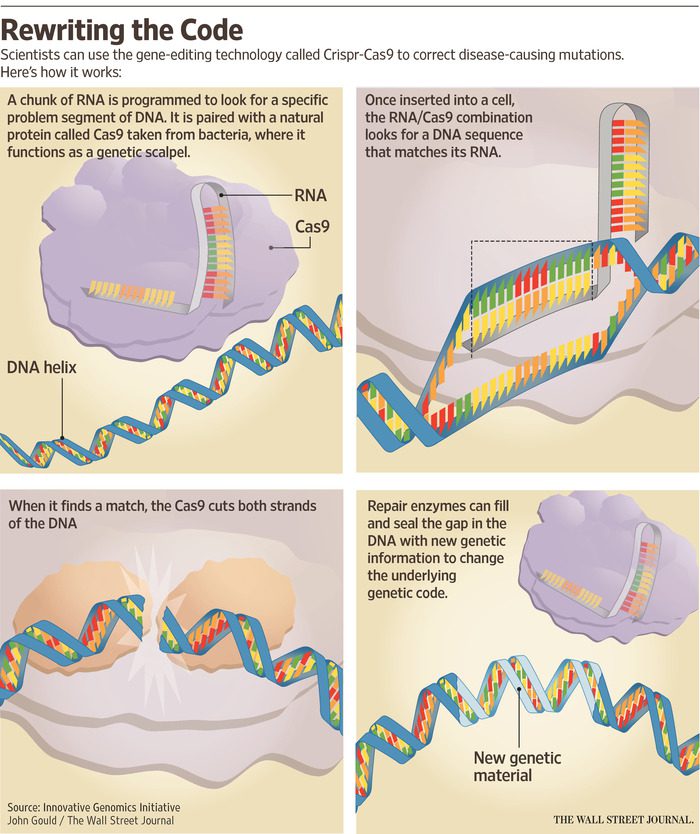
Introduction: In the span of just a few years, CRISPR, an acronym for Clustered Regularly Interspaced Short Palindromic Repeat, has transformed from a novel gene-editing tool to a powerhouse technology reshaping our approach to medicine and genetics. This revolutionary system, likened to genetic scissors, has garnered attention for its ability to make precise changes to…