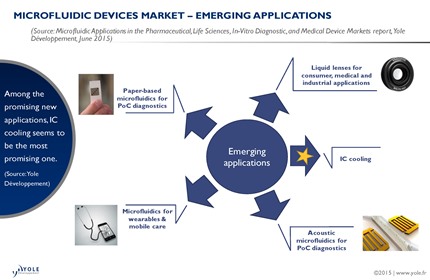
Microfluidics: Tiny Flows Driving Big Revolutions in Medicine, Space, and Security From lab-on-chip diagnostics to implantable drug delivery and Mars exploration, microfluidics is reshaping science and technology at the smallest scales. In the rapidly advancing fields of biotechnology and medicine, microfluidics has emerged as a transformative technology with applications ranging from lab-on-chip diagnostics and implantable…