Pandemics may threaten both internal and external national security – the physical threat to U.S. citizens in terms of morbidity and mortality, and the decreased effectiveness of U.S. armed forces in protecting those citizens from external threats. The economic, political or social turmoil in adversary country is also potential threat. The U.S. military supports U.S. Government responses to public health emergencies such as Ebola, which can cause regional destabilization and spread through global travel, said DARPA. Eliminating pandemic outbreaks and mitigating the impact of a potential high threat biological agent release are national security priorities.
However, recent coronavirus and earlier pandemics have shown that U.S. levels of readiness and global coordination are woefully inadequate. Recent examples of public health emergencies have demonstrated a national and global inability to develop effective preventive or therapeutic solutions in a relevant timescale when an infectious threat emerges. Reviews of recent outbreaks have repeatedly highlighted this capability gap; the significant delay in deployment of solutions during the current coronavirus and earlier Ebola outbreak.
The World Health Organization (WHO) declared a Public Health Emergency of International Concern on 30 January 2020, and a pandemic on 11 March 2020. Since 2021, variants of the virus have emerged and become dominant in many countries, with the Delta, Alpha and Beta variants being the most virulent. As of 11 September 2021, more than 223 million cases and 4.61 million deaths have been confirmed, making it one of the deadliest pandemics in history. By dubbing COVID-19 a pandemic, the WHO is placing it in a different category than several recent deadly outbreaks, including the recent Ebola outbreak in the Democratic Republic of Congo, the Zika virus outbreak in 2016 and the 2014 Ebola outbreak in West Africa. All three of those outbreaks were deemed to be international emergencies. In the last pandemic, the H1N1 influenza virus killed more than 18,000 people in more than 214 countries and territories, according to the WHO. In recent years, other estimates have put H1N1’s toll even higher. A
ll these pandemics have shown that even with all advances in modern medicine we are still not completely safe from pandemics. These epidemic of infectious diseases can spread quickly through human populations across a large region several continents, or even worldwide.
In 2013, DARPA awarded $25 million to Moderna to help establish its messenger RNA platform. That work laid the foundation for the creation of the Biological Technologies Office in 2014 and the Pandemic Prevention Platform (P3) in 2017. In response to the COVID outbreak, Amy Jenkins, the P3 program manager, responded quickly and made awards to four groups to use the newly developed technology to develop a vaccine for COVID-19, which was accomplished in record time and has saved countless lives.
Research into pandemics and diseases sponsored by DARPA early in the last decade is now paying off in the fight against the novel coronavirus, the leader of the agency said July 2020. “We have some examples here around COVID-19 where the investments that DARPA made 10, 15 years ago are ones now at the forefront of providing both interventions, treatments, diagnostics and other ways we’re going to get out of this mess,” Peter Highnam, acting director of the Defense Advanced Research Projects Agency, told reporters
Autonomous Diagnostics to Enable Prevention and Therapeutics, or ADEPT, focused on rapidly identifying pathogens, developing vaccines and quickly ramping up production. “The nucleic acid vaccines that we see today being discussed certainly have strong roots in that work,” he said. Such vaccines inject genetic material such as RNA and DNA into live hosts. The Rapid Vaccine Assessment program finished its work in 2017, but also turned out to be a valuable seed DARPA planted, he said. The program produced an artificial immune system technology to help organizations or companies determine which vaccines to pursue, or not pursue so they can “trim down that early part of the pipeline,” Highnam said.
Meanwhile, the ongoing Pathogen Prevention program has sought the means to help warfighters heading to regions where there might be unknown pathogens. “How do we protect those people health-wise before we go there?” he asked. “How do you rapidly give them something — either as a prophylaxis or something that’s a prevention mechanism? As for the supply chain problem, DARPA’s Make-It program has looked into rapidly manufacturing medications solely from U.S.-based materials in order to mitigate dependence on foreign suppliers. That initiative is not specifically focused on COVID-19, but is looking at domestically making common antibiotics and other supplies used in intensive care units.
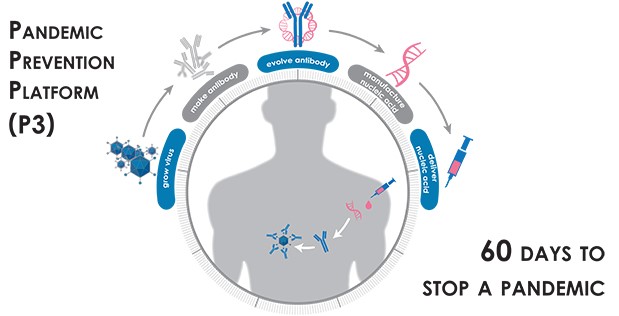
The Defense Advanced Research Projects Agency (DARPA) launched the Pandemic Prevention Platform (P3) program in 2017, with the eventual goal of halting the spread of any infectious disease outbreak before it can escalate into a pandemic. The goal of the Pandemic Prevention Platform (P3) program is to develop an integrated capability to deliver pandemic prevention countermeasures to humans in <60 days. The P3 program aims to revolutionize outbreak response capabilities to allow rapid discovery, characterization, production, and testing of efficacious medical countermeasures. State-of-the-art medical countermeasures often take many months or even years to develop, produce, distribute, and administer. The envisioned P3 platform would cut response time to weeks and stay within the window of relevance for containing an outbreak.
DARPA Seeks to Establish New Platforms for Rapid Development of Medical Countermeasures
The Pandemic Prevention Platform (P3) program aims to support military readiness and global stability through pursuit of novel methods to dramatically accelerate discovery, integration, pre-clinical testing, and manufacturing of medical countermeasures against infectious diseases. P3 confronts the reality that Department of Defense (DoD) personnel are not only deployed around the world for routine operations, but are often among the first responders to outbreaks of emerging or re-emerging disease with pandemic potential (e.g., Ebola).
Advances in medical countermeasures have formed a strong foundation, enabling the creation of a true end-to-end pandemic prevention platform. However, experience gained from conventional responses to emerging infectious diseases has demonstrated that significant bottlenecks hinder the rapid response to an emerging infectious threat,” said Hepburn. “P3 seeks to demonstrate an ability to rapidly produce virus needed to test and evaluate therapies, obtain high potency antibodies within the first weeks of an outbreak, and to scale delivery methods into humans to produce protective levels inside the patient.”
The teams have proven they can meet this ambitious timeline in previous trials using the influenza and Zika viruses. Now they’re being asked to pull off the same feat with the new coronavirus, which more formally goes by the name SARS-CoV-2 and causes the illness known as COVID-19.
Sorrento Therapeutics’s (NASDAQ: SRNE) fully-owned unit SmartPharm Therapeutics has accepted a contract to provide quick protection measures against COVID-19. The contract was granted to SmartPharm by DARPA and is co-supported by JPEO-CBRND. DARPA seeks to speed up the development of an antibody that will support intramuscular injection and provide recipients with potent neutralizing antibodies to mitigate potential SARS-CoV-2 mutations. The DRAPA/JPEO deal will offer support to speed up the creation of Gene MAbs. Gene MAbs is the neutralizing antibody that allows the receiver to produce the protective antibody against SAR-CoV-2.
SmartPharm will receive USD$34 Million through this contract and will use this in Phase II clinical trial of Gene MAbs. SRNE and SmartPharm are jointly working to make plasmid DNA encoding the SARS-CoV-2 neutralizing antibody STI-2020. Currently, the FDA is assessing the IND for STI-2020. It is anticipated that STI-2020 has a high potential and is an ideal candidate for Gene MAbs supply against COVID-19.
DARPA P3 aims specifically to develop a scalable, adaptable, rapid response platform capable of producing relevant numbers of doses against any known or previously unknown infectious threat within 60 days of identification of such a threat in order to keep the outbreak from escalating and decrease disruptions to the military and homeland.
Existing capabilities to respond to an outbreak and develop vaccines and therapeutics often take years or even decades to achieve results. The R&D process for identifying the protective antigens can take months for a simple variant of a known infectious agent, such as seasonal influenza, and years to decades for a newly discovered agent. In many cases, even decades of research have not led to the development of licensed vaccines for known infections.
Further, manufacture of traditional vaccine products typically takes an additional six to nine months. Finally, even when effective vaccines are readily available, immunity in humans can take weeks or months to establish. These solutions often arrive too late—if at all—and in quantities too small to respond to emerging threats. In contrast, the envisioned P3 platform would cut response time to weeks and stay within the window of relevance for containing an outbreak.
We need to be able to move at this speed considering how quickly outbreaks can get out of control,” said Matt Hepburn, the P3 Program Manager. “The technology needs to work on any viral disease, whether it’s one humans have faced before or not.”
The DAPRA approach called for employing antibodies, the proteins that our bodies naturally use to fight infectious diseases, which remain in our bodies after an infection. In the P3 program, the 60-day clock begins when a blood sample is taken from a person who has fully recovered from the disease of interest. Then the researchers screen that sample to find all the protective antibodies the person’s body has made to fight off the virus or bacteria. They use modeling and bioinformatics to choose the antibody that seems most effective at neutralizing the pathogen, and then determine the genetic sequence that codes for the creation of that particular antibody. That snippet of genetic code can then be manufactured quickly and at scale, and injected into people.
Jenkins says this approach is much faster than manufacturing the antibodies themselves. Once the genetic snippets are delivered by injection, “your body becomes the bioreactor” that creates the antibodies, she says. The P3 program’s goal is to have protective levels of the antibodies circulating within 6 to 24 hours. DARPA calls this a “firebreak” technology, because it can provide immediate immunity to medical personnel, first responders, and other vulnerable people.
However, it wouldn’t create the permanent protection that vaccines provide. (Vaccines work by presenting the body with a safe form of the pathogen, thus giving the body a low-stakes opportunity to learn how to respond, which it remembers on future exposures.) “We haven’t taught the body how to make the antibody,” Carnahan says, so the protection isn’t permanent. To put it in terms of a folksy aphorism: “We haven’t taught the body to fish, we’ve just given it a fish.”
Pandemic Prevention Platform (P3) program
P3 focuses on rapid discovery, characterization, production, testing, and delivery of efficacious DNA- and RNA-encoded medical countermeasures, a foundational technology pioneered by DARPA under the Autonomous Diagnostics to Enable Prevention and Therapeutics (ADEPT) program that provides the body with instructions on how to immediately begin producing protective antibodies against a given threat.
A principal benefit of the nucleic-acid-based approach to limiting the spread of infection is that genetic constructs introduced into the body would process quickly and not integrate into an individual’s genome. Similarly, the antibodies produced in response to treatment would only be present in the body for weeks to months. This is consistent with DARPA’s intent to safely deliver transient immunity, halting the spread of disease by creating a firewall, and buying time for longer-term medical responses to be developed and deployed.
Robert Carnahan, who works with Crowe at the Vanderbilt Vaccine Center, explains that their method offers only temporary protection because the snippets of genetic code are messenger RNA, molecules that carry instructions for protein production. When the team’s specially designed mRNA is injected into the body, it’s taken up by cells (likely those in the liver) that churn out the needed antibodies. But eventually that RNA degrades, as do the antibodies that circulate through the blood stream.
Key to this undertaking are nucleic-acid-based technologies—those that are centered on DNA and RNA—including some developed under DARPA’s Autonomous Diagnostics to Enable Prevention and Therapeutics (ADEPT) program. Using these tools, scientists can identify protective antibodies from recovering patients and then, through a biological version of reverse engineering, manufacture genetic constructs that, when delivered, can instruct an individual’s body to produce similar protective antibodies. Significant quantities of these nucleic acid “blueprints” can be rapidly manufactured compared to state-of-the-art antibody production methods.
The P3 program seeks to unlock the potential of these coded genetic constructs—establishing them as the basis for a threat-agnostic platform technology—by achieving and integrating breakthroughs in three key areas: novel approaches for the growth of viruses for use in testing and evaluation of countermeasures; rapid identification and maturation of protective antibodies to increase their potency; and novel technologies for the delivery of nucleic acid constructs into patients to encode the antibody of interest and produce a protective response.
What is required now are breakthroughs in three other technology areas to bridge those past DARPA achievements and overcome the remaining bottlenecks that hinder rapid response to pandemic threats. The P3 program will pursue innovations in those three areas:
On-demand platform to grow virus: First, proposed approaches must be capable of rapidly producing viruses in sufficient quantity for countermeasure development from discovery through testing, potentially by growing viruses in engineered cell types or through similar rapid processes. Although the BAA targets pathogens generally, successful offerors will be expected to work with viruses in performing contemplated activities.
System to evolve antibodies: rapid creation of highly potent medical countermeasures: Second, proposed approaches must be capable of rapidly producing high-potency antibodies or other proposed biological products, with an expectation that offerors will likely identify an in vitro antibody maturation platform. Although DARPA is willing to consider a range of biological products that target particular viruses—including other proteins, peptides, and oligonucleotides—the BAA assumes that antibodies will be used to ensure rapid countermeasure development.
Delivery of medical countermeasure(s): reproducible effects which lead to protective levels of product.
Third, proposed approaches must identify delivery methods that are designed to induce in animal models complete protection against a targeted pathogen within three days, ideally following a single administration using a method that can be employed in austere conditions and with a protection period lasting more than thirty days.
P3 performers are expected to demonstrate safety of their nucleic acid product against one target pathogen in a phase I clinical trial. Ultimately, the performer teams will be evaluated on the results of their clinical trial and their ability to complete the end-to-end process within 60 days from the time the pathogen-containing sample is first obtained. Also, to demonstrate the broad utility of the platform, each team will target a variety of viral pathogens including influenza, chikungunya, MERS-CoV, and Mayaro virus, among others.
P3 Program awards
The institutions funded through the P3 program include: MedImmune, Abcellera Biologics Inc., Duke University and Vanderbilt University
ABCELLERA announces partnership with NIAID Vaccine Research Center and ICHOR medical Systems to tackle pandemic Viral Outbreaks
As part of the four-year, USD $30 million project, NIAID Vaccine Research Center and Ichor will contribute world-leading expertise in virology, vaccinology, nucleic acid antibody vectorization and delivery to complement AbCellera’s capabilities. Together, the team will build an end-to-end platform capable of developing field-ready medical countermeasures within 60 days of a viral outbreak.
Central to AbCellera’s platform is a microfluidic technology that allows for deep mining of natural immune responses, yielding large and diverse panels of antibodies. AbCellera’s technology has unprecedented throughput and supports a variety of miniaturized assays to directly select antibodies produced by single cells from any species, including humans. In the P3 project, these capabilities will be used to screen millions of immune cells from human patients previously exposed to infectious pathogens and isolate panels of potent neutralizing antibodies as candidate therapeutics for pandemic response.
In 2018, as part of a first simulated pandemic exercise, AbCellera performed rapid antibody discovery from camelids infected with Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) which has high pandemic potential. This effort, in collaboration with Colorado State University, identified thousands of MERS-CoV binding antibodies in a single afternoon. The first 355 unique sequences, including many antibodies that are potent virus neutralizers, were obtained in a mere three days and 19 hours.
Camels act as a natural reservoir of MERS-CoV and exhibit mild symptoms when infected. However, when the virus spreads to humans, it causes severe respiratory illness that is fatal in about 35% of infections (60% in hospital settings) and can be spread through the air when infected individuals cough. It was first reported in Saudi Arabia in 2012.
“We are proud of the people on our phenomenal AbCellera team, who approached this simulation with the urgency of a real pandemic outbreak, working in shifts around the clock to meet the goals set out by this challenge,” said Ester Falconer, P3 Project Lead and Group Leader at AbCellera. “By using AbCellera’s rapid antibody discovery technology, we were able to complete the screening portion in just six hours and execute every step seamlessly to achieve the end goal ahead of schedule. To the best of our knowledge, this is unprecedented speed for an antibody campaign, and we’re optimistic that this approach could clear a major bottleneck in halting real pandemic outbreaks.”
Now, one year into this four-year project, AbCellera, the NIAID Vaccine Research Center, and Ichor will be testing how quickly they can move from discovering broadly-neutralizing antibodies against influenza virus to delivering a nucleic acid-based countermeasure that can protect against infection. For this, the expertise of the NIAID Vaccine Research Center and Ichor is critical. In this expanded challenge, the team will discover potent antibody neutralizers of pandemic influenza, incorporate these sequences into nucleic acid-based therapeutics, use Ichor’s TriGrid electroporation technology to deliver the nucleic acids into animals, and perform in vivo tests to demonstrate the therapeutics’ ability to act as a prophylactic against infection. This represents a paradigm shift in antibody treatments, since delivering nucleic acids that code for a neutralizing antibody directly to the patient can bypass the difficult and time-consuming manufacturing process conventionally required for therapeutic antibodies. Nucleic acid-based treatments have the potential to be deployed more rapidly than conventional countermeasures in response to an emerging pandemic.
“AbCellera’s team has made tremendous strides towards building the first technology platform capable of rapid pandemic response. DARPA’s vision of deploying anti-viral countermeasures in 60 days, which was widely perceived as science fiction, is fast becoming a reality,” said Carl Hansen, CEO of AbCellera. “We are excited to pressure-test our platform in a simulated flu pandemic and are confident the combined expertise and technology of the NIAID Vaccine Research Center, Ichor, and AbCellera is up to the task. In addition to setting new speed and performance records for human antibody discovery, this exercise has the potential to yield highly-potent human antibodies for development as therapeutics and prophylactics.”
Duke funded for DARPA Pandemic Prevent Platform (P3)
The Duke Human Vaccine Institute has received a $12.8 million, 30-month grant from the U.S. Department of Defense, Defense Advanced Research Projects Agency (DARPA) to develop a system capable of halting viral pandemics within 60 days. The program, called DARPA Pandemic Prevention Platform (P3), seeks to combine expertise in virology, immunology and clinical manufacturing to rapidly identify and respond to disease outbreaks such as SARS, pandemic influenza and Zika before they spread widely.
Biologic countermeasures, such as monoclonal antibodies (mAbs), are a rapid/effective means of controlling and containing outbreaks of emerging pathogens where no licensed therapeutic or vaccine is available. However, standard paradigms for their production and delivery limits their use as a first-line prophylactic or therapeutic, since the process for their identification, optimization and production can take months to years. The Defense Advanced Research Projects Agency (DARPA) has recently funded Duke as part of their Pandemic Prevention Platform (P3) to address these challenges.
The overall US Department of Defense DARPA program aims to develop an integrated technology platform that gives public health officials the capability to halt the spread of any viral disease outbreak within 60 days, before it can escalate to pandemic status. In contrast with state-of-the-art medical countermeasures, which typically take many months or even years to develop, produce, distribute, and administer, the envisioned P3 platform would cut response time to weeks and stay within the window of relevance for containing an outbreak.
The Duke DARPA Pandemic Prevention Platform (P3) team seeks to apply its experience, innovations, cutting-edge research portfolio, and in-house cGMP manufacturing capabilities to greatly expedite mAb countermeasures for future pandemics. The fully integrated platform will be a major advancement in rapid pandemic countermeasure development and will address the significant global challenge pandemic outbreaks have on both civilian and military populations.
The Duke DARPA P3 program (DARPA agreement number HR0011-17-2-0069), under the leadership of Dr. Gregory Sempowski, will combine world-class expertise in virology, immunology and CGMP manufacturing to create a fully integrated platform capable of responding to a viral pandemic. For this program, we are focusing on three main areas of innovation to development platform to be applied in future pandemics:
1. On-Demand Platform to Grow Virus: Develop methods to support viral propagation, so that virus can be used for downstream studies
2. System to Identify and Evolve Antibodies: Isolate neutralizing antibodies from convalescent PBMC and plasma, improve the antibody 100-fold in function by in vitro antibody evolution and transfer antibody sequence for production and delivery optimization/testing
3. Deliver Medical Countermeasures: Develop an mRNA-based medical countermeasure with optimal potency, half-life and ease of use in the field
Vanderbilt University Medical Center (VUMC)
DARPA awarded VUMC a grant worth up to $28 million to join a groundbreaking effort to develop new rapid response platforms for the discovery and delivery of antibodies to fight global viral threats like Zika and Ebola.
“Developing an integrated pipeline technology for identifying ultrapotent human antibodies and accomplishing rapid delivery … could revolutionize how antiviral interventions are conducted,” the DARPA agreement states.
VUMC’s approach addresses these goals by vastly speeding up the antibody discovery, validation, and delivery processes. This accelerated process bypasses much of the cell-based work of their standard approach, and directly defines the genetic determinants of highly potent anti-viral antibodies directly from the serum of survivors. Numerous other innovations have also been coupled to this workflow to dramatically speed up the therapeutic candidate downselection and selection processes.
Yet this process still took many months, or even years if it was a sufficiently complex challenge, Robert Carnahan, Associate Professor of Pediatrics at the Vaccine Center at VUMC, explained. It’s expected that the lab will markedly improve its timeline for developing these therapeutics. “We had picked three different viruses and the last one will be unknown to us. They will give it to us and we’ll simulate a live event,” said James Crowe Jr., MD, director of the Vanderbilt Vaccine Center. “So it’s basically broken down into four sort of sprints, and we’ll try to get faster and faster. It’s kind of like athletic training.” VUMC’s agreement with DARPA states that the funding program will include four attempts over five years to successfully achieve the two-month response time.
Fighting what is essentially a race against time in speeding up the development of anti-viral antibodies, VUMC scientists enlisted Twist Bioscience in order to use its high-throughput platform to allow them to more quickly scale their antibody discovery process. Carnahan said the lab, using Twist Bioscience genes, has “discovered a way to markedly speed up the antibody discovery process.”
In a new approach to their human antibody screening, bioinformatics on the disease survivor’s B-cells separates out interesting antibody candidates. “From the pool of tensof-thousands of antibodies in one patient, we can narrow it
down bioinformatically to around 1,000 candidate sequences,” Carnahan explained. “We can then get synthetic genes for these candidates from Twist at an unprecedented speed and scale. We utilize these constructs in our recombinant antibody workflow to produce material for screening and selection in a matter of days. Combining the data from this screening process with our in-house bioinformatic analyses leads to identification of lead candidates, and helps us understand conserved characteristics of potent anti-viral antibodies.”
Importantly, VUMC’s approach is turning to pure synthetic biology, using recombinant systems to generate these important pharmaceuticals. By the very nature of their approach, synthetic DNA is essential to develop a process that worked as quickly and reliably as possible at a molecular level. VUMC chose to utilize synthetic DNA supplied by Twist Bioscience, as the company offers a highly scalable gene synthesis platform, where DNA is synthesised on silicon. High-throughput, affordable synthetic DNA from Twist Bioscience therefore allows researchers to accelerate the antibody identification process.
Jenkins says that all of the P3 groups (the others are Greg Semposki’s lab at Duke University, a small Vancouver company called AbCellera, and the big pharma company AstraZeneca) have made great strides in technologies that rapidly identify promising antibodies. In their earlier trials, the longer part of the process was manufacturing the mRNA and preparing for safety studies in animals. If the mRNA is intended for human use, the manufacturing and testing processes will be much slower because there will be many more regulatory hoops to jump through.
Crowe and Carnahan’s team has seen another project through to human trials; last year the lab worked with the biotech company Moderna to make mRNA that coded for antibodies that protected against the Chikungunya virus. “We showed it can be done,” Crowe says. Carnahan says the team has been scrambling for weeks to build the tools that enable them to screen for the protective antibodies; since very little is known about this new coronavirus, “the toolkit is being built on the fly.” They were also waiting for a blood sample from a fully recovered U.S. patient. Now they have their first sample, and are hoping for more in the coming weeks, so the real work is beginning. “We are doggedly pursuing neutralizing antibodies,” he says. “Right now we have active pursuit going on.”
Even if everything goes perfectly for Crowe’s team and they have a potent antibody or mRNA ready for manufacture by the end of April, they’d have to get approval from the U.S. Food and Drug Administration. To get a therapy approved for human use typically takes years of studies on toxicity, stability, and efficacy. Crowe says that one possible shortcut is the FDA’s compassionate use program, which allows people to use unapproved drugs in certain life-threatening situations.
Touchlight and Vanderbilt University Medical Center collaborate in July 2021
Touchlight, the leading synthetic DNA manufacturer, is collaborating with the Vanderbilt Vaccine Center (VVC) at Vanderbilt University Medical Center as part of the DARPA P3 programme.
The collaboration will explore the use of synthetic dbDNA to deliver antibody-based prophylaxis against pandemic disease threats. The aim is to make such therapies available in a significantly shorter timeframe by avoiding the long and costly large-scale production requirements of both conventional antibody-based treatments and plasmid DNA manufacture. Unlike vaccines, prophylactic antibodies have an immediate neutralising effect against the virus and may be able to act as a vaccine alternative or supplement whilst immunity builds.
Touchlight will develop and test a panel of novel dbDNA designs that encode for anti-Zika antibodies identified by Vanderbilt with the goal of boosting expression of the antibody to therapeutic levels. The project will also investigate a variety of delivery mechanisms including electroporation and targeted nanoparticles. The combination of these two aspects is aimed to deliver an improved expression profile in patients that enables rapid onset of protective levels of antibody.
Touchlight’s unique synthetic DNA vector, brings multiple advantages over traditional plasmid DNA manufacture and other nucleic acid manufacturing techniques, including speed, reliability and scalability. Touchlight’s dbDNA platform could unlock the potential for prophylactic nucleic acid launched antibodies, where the demands on rapid scale up to protect a population are equal or greater than those of vaccines.
Sarah Moore, Director of Gene Therapy Research at Touchlight said: “Antibody treatments can be the key to a swift pandemic response, if they can provide a good level of protection at short notice, until vaccines are available. We believe that the use of dbDNA to generate antibodies in vivo has the potential to significantly accelerate the availability of such therapies.”
Robert Carnahan, Associate Director at the Vanderbilt Vaccine Center, said:
“The COVID-19 pandemic has made clear that the most significant bottleneck to rapid antiviral antibody therapeutics is the speed and scaling of manufacturing and delivery. Nucleic acid-delivered antibodies are a vital need in our pandemic fighting arsenal to directly address this bottleneck. Touchlight dbDNA’s technology presents the possibility of addressing these pressing needs in therapeutic antibody manufacturing speed and scalability.”
References and resources also include: