Rechargeable lithium-ion batteries have been workhorse of the consumer electronics market including portable electronics, implantable devices, power tools, and hybrid/full electric vehicles (EVs) due to their ability to store large amounts of energy per unit weight and per unit volume, low self-discharge rate, long cycle life. They are also relatively maintenance-free and contain fewer toxic chemicals than other batteries.
Batteries are also critical for military missions since mission success and soldiers’ lives often depend directly on a military battery’s performance. The expected improvements in energy density may enable advances in directed energy weapons, increase the loiter time of unmanned vehicles, lead to more effective sensors, and reduce the size and weight of manportable systems.
Batteries are of two types, the primary batteries that are single use batteries, once they are discharged, they must be discarded. Rechargeable batteries are generally referred to as secondary batteries. Lithium-ion battery or li-ion battery (abbreviated as LIB) is a type of rechargeable battery in which lithium-ions move from the negative electrode to the positive electrode during discharge and from positive electrode to negative electrode while charging. Lithium-ion battery (LIB) consists of a graphite electrode (anode), an electrolyte (usually a lithium salt), and a metal oxide electrode (usually an oxide containing lithium).
Lithium-ion batteries have large energy density of 372 mAh/g, hundreds of cycle of durability, and are routinely packed into mobile phones, laptops and electric cars. Global lithium-ion battery market was valued at $ 24.5 billion and is projected to grow at a promising CAGR of 15% to reach $ 56 billion by 2024 on account of increasing demand for energy-efficient, safe and low-cost batteries.
For widespread adoption of electric cars, their range needs to be increased which require dramatic improvement in battery energy density and cycle durability as well as decreasing their cost. These next generation of batteries that are able to fully charge more quickly, and produce 30%-40% more electricity than today’s lithium-ion batteries, could help transform the electric car market, allow the storage of solar electricity at the household scale and power the medical implantable devices.
The more energy a battery has, the further a car can go. However Li-ion batteries are reaching their energy density limit, that also hastens the pace of degradation and shortens battery life. Several higher-density batteries don’t have stable chemical compositions either, leaving them dangerously vulnerable to combustion. In 2016, South Korean giant Samsung called for complete re haul of its latest flagship device- Galaxy Note 7 after consumers around the globe reported their handsets exploding and causing damages. One of the main causes of catching fire appears to continuous increase in energy density of Lithium-ion battery units driven by increased user requirements like full HD, and high speed leading to large processing requirements of multi-core CPUs and increasing desire to produce sleeker design . Since launching in 2018, Hyundai Motor Co. has reported several blazes in its Kona electric vehicle related to the battery and previously recalled some of the cars to do a system update.
Because of these safety issues Researchers are developing many promising new battery chemistries to replace Lithium-ion. Lithium-ion batteries also are also costly and limited resources of lithium may restrict their further application and hence demands urgent development of the low-cost batteries based on new energy storage chemistries. New batteries are also required to satisfy the increasing demands of high-performance, energy storage devices to power electric vehicles, smart homes, smart phones, and even smart wearables, that will be safer and, eventually, cheaper.
Research firm IDTechEx estimates that advanced and post-lithium-ion battery technologies will achieve a market value of $14bn in 2026, comprising about 10 per cent of the entire battery market. However there is need to develop efficient manufacturing processes, enhance durability and safety and reduce the costs before consumers start using these non-traditional batteries.
New Battery technologies
We’re on the verge of a power revolution with plethora of battery discoveries coming into commercial domain soon. Researchers from universities, battery manufacturers and automobile makers are constantly trying to find the optimum combination of negative and positive electrode materials to make a lightweight, cost-effective, high-capacity battery. Some of the promising batteries are Lithium-air breathing and Aluminum Air batteries, Gold nanowire batteries, titanium dioxide anode battery, Silicon and Germanium Nanowire, Solid state and Graphene batteries.
Solid state batteries
In solid-state batteries the liquid electrolytes normally used in conventional lithium-ion batteries are replaced with solid ones, which make it possible to replace conventional electrodes with lithium metal ones that hold far more energy. Doing away with the liquid electrolyte, which is flammable, can also improve the safety of batteries, which leads to cost and size savings, particularly in electric vehicles, by reducing the need for complex cooling systems.
The result is a battery that can operate at super capacitor levels to completely charge or discharge in just seven minutes – making it ideal for cars. Since its solid state that also means it’s far more stable and safer than current batteries. The solid-state unit should also be able to work in as low as minus 30 degrees Celsius and up to one hundred. Researchers at Toyota and Tokyo Institute of Technology said they had developed solid-state batteries with more than three times the storage capacity of lithium-ion state batteries. Hitachi Zosen of Japan says it plans to commercialize the technology by 2020, but acknowledges it has yet to work out the manufacturing process.
One approach with huge potential is using lithium-metal as the anode in place of the graphite and copper currently used could significantly boost the density of today’s batteries, enabling them to run far longer and hold far more energy. The problem is safety. As the battery is charged, growths known as dendrites tend to form on the surface of the lithium metal anode, causing electrical shorts, fires and ultimately the failure of the device.
We saw a few innovative approaches to solving this problem in 2020, including one from scientists at Washington State University whose approach to preventing dendrites involved adding a few key chemicals to the cathode and electrolyte solution. This led to the formation of a protective layer on the surface of the lithium metal anode, enabling it to remain stable while being charged across 500 cycles. Working in the team’s favor as it eyes commercialization is that the process can be integrated into existing manufacturing procedures.
In February 2020 , a group of researchers at University of California San Diego built a tiny ultrasound device and incorporated it into a lithium-metal battery, enabling it to send high-frequency sound waves through the liquid electrolyte as a way of causing it to flow gently rather than remain static. This had the effect of creating neat, uniform distribution of lithium on the anode rather than the uneven clumps that usually form and lead to dendritic growth. In testing, this ultra-sound equipped battery could be charged from zero to 100 percent in just 10 minutes, and proved stable across 250 charging cycles, again boding well for safety.
Californian battery-maker QuantumScape
In December 2020, Californian battery-maker QuantumScape announced some performance numbers for its solid-state lithium-metal battery designed for use in electric vehicles, It’s claimed to add as much as 80 percent to the range of an electric car, and charge from 0-80 percent in just 15 minutes. Using a solid electrolyte instead of the typical liquid solution, solid state batteries can store considerably more energy by weight and volume than today’s lithium cells, but creating one that can stand up to the rigors of use in an electric vehicle – high charge and discharge rates, long lifespans, temperature and safety concerns – has proven difficult.
The company claims that indeed it could, thanks in part to the use of a solid electrolyte rather than a liquid one, and an anode made from lithium metal that forms itself around the current collector as the battery is charged. This solid state lithium-metal battery also avoids the dendrite problem thanks to a separator between the anode and cathode made from solid ceramic material. Energy density is reportedly excellent.
In volumetric terms, the new battery can store 1 kWh/liter, about four times what the current Tesla Model 3 battery stores. By weight, it offers somewhere between 380-500 Wh/kg, compared to around 260 Wh/kg for the current Tesla packs – although renowned optimist Elon Musk reckons he can get 400 Wh/kg out of Tesla cells within three to four years in serious volume. The battery was also found to retain 80 percent of its capacity after 800 cycles, which bodes well for its safety and longevity.
Tesla Battery breakthrough unveiled in Sep 2020
In Sep 2020, Tesla CEO Elon Musk has unveiled a series of battery technology breakthroughs, which he claims will revolutionise electric cars. A new shingled spiral design of Tesla’s lithium-ion battery will transform the way energy can be stored and charged within a lithium-ion battery, offering five-times more energy, a 16 per cent increase in range and 500 per cent more power. The advances will make car batteries 56 per cent cheaper and will enable the production of Tesla’s first truly affordable vehicle.
Dash of graphene leads to “toughest” solid battery electrolyte to date, reported in June 2020
A solid-state battery, where the liquid electrolyte that carries the charge is swapped out for a solid alternative, promises a number of performance benefits over today’s solutions, but there are a few problems to solve first. But integrating a solid electrolyte isn’t exactly easy, with efforts so far often plagued by fracturing and corrosion of other parts of the battery. Using ceramic materials has shaped as one option, but their brittle nature has also proved problematic.
The Brown University researchers believe they can overcome this drawback by adding a dash of graphene, the strong and lightweight wonder material that also offers high electrical conductivity, an attribute that had to be managed carefully for these purposes. Scientists at Brown University are reporting a new design that overcomes some of the key hurdles, using a delicate mix of ceramics and the wonder material graphene to produce the toughest solid electrolyte to date.
Inolith, a Swiss startup, say Electric car batteries with 600 miles of range using inorganic solvents
Inolith, a Swiss startup, says its new high-density lithium-ion batteries can do just that. The company claims to have made the world’s first 1,000 Wh/kg rechargeable battery. (Watt-hours per kilogram is a unit of measurement commonly used to describe the density of energy in batteries.) By comparison, the batteries that Tesla uses in its Model 3 — the so-called 2170 cells — are an estimated 250 Wh/kg; the company plans to eventually push that to 330 Wh/kg. Meanwhile, the US Department of Energy is funding a program to create 500 Wh/kg battery cells.
Innolith’s design is primarily based on the replacement of organic solvents (which are inherently flammable) with an inorganic substance that is less flammable and considerably more stable. Organic materials are highly reactive and, over time, this makes the batteries ineffective. With non-organic salt-like materials, the system is able to pack in a lot more energy without becoming unstable.
“We take the organic materials out and replace them with inorganic or basically salt-like materials, and that does two things for you,” Greenshields says. “One is it gets rid of your fire risk, so, of course, there’s nothing to burn. And the second part is you’ve also got rid of the most reactive components in the system, which makes it easier to build a battery where you can pack in a lot of energy without the thing becoming unstable.The organic materials found in most lithium-ion batteries are the “principle source of side reactions,” which, over time, can consume the active materials in the battery and turn the whole closed-loop system into something “non-productive,” he adds. Innolith claims its new battery has done away with this problem.
Innolith says it will bring its innovative new battery to market via an initial pilot production in Germany, followed by licensing partnerships with major battery and automotive companies. (Greenshields cited India as one country that could be interested in Innolith’s technology.) Development and commercialization will likely take three to five years, which means the company’s battery won’t be ready to go to market until 2022 at the earliest.
Another breakthrough reported is by Jeff Dahn in the Journal of The Electrochemical Society (JES), where he announced to the world that Tesla may soon have a battery that makes their robot taxis and long-haul electric trucks viable. Dahn and his research group are Tesla’s battery research partner. Dahn said: “Cells of this type should be able to power an electric vehicle for over one million miles and last at least two decades in grid energy storage.”
In the article, Dahn and his research team provide full details of the new cell to create a benchmark for further research. They used a cathode material from the family of Ni rich NCM cathode materials. It has a specific capacity which is 20 percent higher than that of the cathodes used in Li-ion batteries that power today’s mobile electronic devices.The cathode material chosen, NCM 523 (50 percent Nickel, 20 percent Cobalt, 30 percent Manganese), is stable and an excellent reference and starting point for further developments. Other key components explored were graphite anodes and blends of solvents, additives, and salt for the electrolyte solutions.
Aurbach said the batteries described in the paper can be used for electric vehicles right away. “However, since the goal of the study was to provide a reliable benchmark and reference for Li-ion battery technology, the specific energy density of the batteries described is not the highest compared to what can be really reached by advanced Li-ion batteries. Based on the study, Li-ion batteries will soon be developed that make driving over 500 kilometers (over 300 miles) from charge to charge possible.”
Chinese company CATL
Chinese company CATL claims it is at the forefront of electric vehicle technology, supplying its technology to Volvo, BMW, and Tesla. In August 2020, the Chinese company claimed it is developing a battery with no nickel or cobalt, according to a report from news outlet Reuters. Reducing a battery’s reliance on the metals means the company can theoretically produce a battery capable of lowering the cost of electric vehicles. If cheap enough, it could bring the cost of electric vehicles down to a level comparable with traditional internal combustion vehicles. In another story from Reuters reported the company is developing batteries designed to fit within a car’s chassis, allowing the battery casing to be discarded – saving weight and improving efficiency, as well as increasing the number of battery cells in a car.
It has completed development of a battery that it claims will last for 2 million kilometres of driving. In an interview with news agency Bloomberg earlier this week, CATL’s chairman Zeng Yuqun said development of the battery pack was finished and production was ready to begin. “If someone places an order, we are ready to produce,” Mr Yuqun told Bloomberg. The new CATL battery pack is expected to last 16 years of use. By comparison, the most common battery warranty currently offered on an electric car in Australia is eight years or 160,000km.

(a) Theoretical capacities of Li metal, graphite (LiC 6 ), and Mg-metal anodes. (b) Elemental abundance in the earth’s crust. (c) Operational voltage and speci fi c capacity of a Mg battery compared with other electrochemical power sources.
Silicon Anodes
Silicon and Germanium are two candidates that can replace the traditional graphite anode to increase the energy density and cycle durability of lithium-ion batteries. Compared to the standard graphite anode, silicon can bond 25 times more with Li-ions, increasing the battery density by 30% or even more. Current battery designs contain just 1 to 5% silicon.
Even though Silicon has the highest known theoretical specific capacity of any material (~3600 mAh/g), it has limited use as an anode in lithium ion batteries due to the mechanical instability caused by the large volume expansion as silicon holds up the Li-ion, and when energy is consumed it shrinks, leading to a degradation of the battery. Like silicon, germanium too doesn’t handle charging very well, it expands during charging and disintegrate after a small number of cycles.
Material scientists are working on mechanisms for overcoming this issue, and some of the innovations in silicon-based anodes are already finding their way onto the market. The solutions are tested and proven for high-energy density, ultra-fast charging and wide temperature tolerance. With such batteries, a vehicle can be charged enough in just 5 minutes to cover distances of 400km – almost eight times faster charging than most batteries available today.
A KAIST research team led by Professors Jang Wook Choi and Ali Coskun reported a molecular pulley binder for high-capacity silicon anodes of lithium ion batteries in Science in July 2017. Another KAIST team in 2020 proposed rational encapsulation strategy of a silicon–carbon (Si–C) composite as a high-performance anode material for lithium-ion batteries (LIBs). The Si–C composite material is prepared via a one-pot hydrothermal method by using silicon nanoparticles modified using an etching route and sucrose as a carbon precursor.
The most commonly used approach to improve lithium-ion flow speeds is to make electrode particles nanometers in size to shorten the amount of distance that lithium ions have to travel. However, there are many challenges to this approach. Nanoparticles can prove difficult to pack tightly together, limiting the amount of energy they can store per unit volume. They can also result in more unwanted chemical reactions with electrolytes compared with regular electrode materials, so such batteries do not last as long. Moreover, nanoparticles can also prove complex and expensive to make.
Sodium-Ion
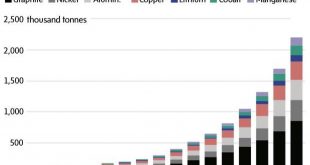
Because billions of electronics rely on lithium-ion technology, there’s growing concern over long-term lithium shortages. Lithium is the 33rd most abundant element in the Earth’s crust. Additionally, large amounts of cobalt—which are right above lithium in terms of abundance—are needed to form electrodes. In fact, four years ago, an MIT study found that cobalt demand could outpace global supply by 1.6 times. Sixty percent of cobalt stores reside within the Democratic Republic of the Congo—where mining is linked to child labor. In addition to these ethical concerns, companies must also decide if cobalt’s $33,000-per-ton cost is a sustainable solution.
Sodium is many magnitudes more abundant than lithium—ranked 6th overall. It’s found everywhere in the Earth’s crust and is harvestable from the ocean. Because Na-ion batteries don’t require cobalt electrodes, they are also considerably more affordable.
Sodium-ion (Na-ion) batteries don’t represent a massive departure from lithium-ion variants. The elemental structure of sodium is quite similar to lithium (being a Group 1 metal), and thus material testing processes are similar. Manufacturing processes are comparable, too. Recent research developments (like those to increase sodium storage behavior via electron-rich element-doped amorphous carbon) are aiming to make Na-ion batteries a more affordable and sustainable replacement to Li-ion batteries.
Starting with recyclable PET plastic, scientists at Purdue University were able to reduce the material to flakes, which were then treated with ultrafast microwave irradiation to turn them into something known as disodium terephthalate. This small organic molecule has long been fancied as a potential anode material due to its excellent electrochemical performance, and the team was able to demonstrate its creation as part of a functioning sodium-ion cell, reported in April 2020. “We are helping to address the growth in the proliferation of renewable energy conversion and storage, which stems from the societal attention and increasing awareness of climate change and energy resource limitation,” lead researcher Vilas Pol said at the time.
Fluoride-ion batteries
In 2018, Honda said that it has made a breakthrough in battery technology. The Japanese automotive manufacturer, in collaboration with the California Institute of Technology (Caltech) and NASA’s Jet Propulsion Laboratory (JPL), developed a new battery chemistry called fluoride-ion which demonstrates better performance than lithium-ion batteries, which are currently used, while causing less damage to the environment. Honda says that the fluoride-ion batteries offer energy density that is 10 times greater when compared to lithium-ion batteries. This translates into a greater ability to store more electricity in a given volume, and thus greater range for an electric car. There will be no need to make the battery pack bigger. There is also no risk of overheating and the new technology does not call for the use of rare metals like cobalt and lithium, the cost of which can be extremely volatile.
The high performance of the battery is thanks to the low atomic weight of fluorine, which is the key component of the battery. The one drawback with the use of fluorine-ion batteries was that the batteries needed temperatures of around 150 degrees Celsius (302 degrees Fahrenheit) to work. Honda’s breakthrough was finding a way to make these batteries work at room temperature by using a new fluoride electrolyte that the researchers developed. Honda says the technology has been tested successfully in the lab, but it still needs to be commercialized successfully.
A team of researchers from Kyoto University and Toyota Motor have developed new fluoride-ion battery which would hold about seven times as much energy per unit of weight as conventional li-ion batteries, could allow electric vehicles to run 1,000 km on a single charge. The team reported in August 2020 that it has developed a prototype rechargeable battery based on fluoride, the anion — the negatively charged ion — of elemental fluorine. A fluoride-ion battery, or FIB, generates electricity by shuttling fluoride ions from one electrode to the other through a fluoride-ion-conducting electrolyte.
Mineral Petrovit Discovered By Russian Scientists, Can Be Used as cathode In Rechargeable Batteries
In Nov 2020, A team at the University of St. Petersburg, led by Stanislav Filatov, a specialist in crystals (crystallographer) dug out petrovite, aka Na10CaCu2(SO4)8 which is a blue globular and gaseous mineral that can power the batteries that can be stored as a form of renewable source of energy. The newly discovered tabular crystals are composed of oxygen atoms, sodium-sulfur, and copper, that form a porous structure.
According to research published in the Mineralogical Magazine, the recent find by the scientists is a breakthrough as the mineral promises ionic conductivity that can be used as a cathode material for sodium-ion batteries. Scientists have conducted research for the petrovite for nearly 40 years. The mineral was observed by a research associate Svetlana Moskaleva at the Institute of Volcanology and Seismology of the Far Eastern Branch of the Russian Academy of Sciences. The mineral promised the scope for running futuristic alternative technology for energy storage systems. Furthermore, scientists termed the discovered mineral a “low cost’ sodium in the Earth’s crust that can take renewable energy to new heights with its peculiar molecular hallmark. At present, the biggest problem for this use is the small amount of a transition metal—copper—in the crystal structure of the mineral. It might be solved by synthesizing a compound with the same structure as petrovite in the laboratory,” said researcher Filatov.
Zinc Air
Zinc-air batteries have long-been thought of as a safer, cheaper and more sustainable replacement to lithium-ion. The heavy metal is one of the world’s most abundant and more environmentally friendly to extract than lithium, but since zinc-air batteries were first produced in the 1930s they have only been single use, powering devices like hearing aids.
However a team from the University of Sydney’s faculty of Engineering and IT have developed a new way to recharge the batteries using a three step method. Lead researcher Professor Yuan Chen says they found a way to control the composition, size and crystallinity of iron and cobalt, creating bifunctional oxygen electrocatalysts – in other words – react to increase and decrease the amount of oxygen needed to recharge the battery. Their capacity to store five times more energy than current models makes the batteries suited for powering electric cars and other long lasting devices. Professor Chen said despite the excitement over the find, the technology will take time to perfect, with issues including a limited 120 recharge cycle compared to lithium-ion’s 400-1200 range, and a slow energy delivery speed.
A new battery design could change that. By tweaking the building materials, researchers created a prototype of a zinc-air battery that could be recharged hundreds of times. Such long-lasting devices, described in the Jan. 2021 Science, could one day power electric cars or other electronics. Sun and colleagues built a zinc-air battery using a new electrolyte that contains water-repellant ions. Those ions stick to the cathode, preventing H2O from the electrolyte from reacting with incoming oxygen at the cathode surface. As a result, zinc ions from the anode can travel to the cathode and react directly with oxygen from the air. This relatively simple reaction is easy to run backward to recharge the battery. What’s more, the new electrolyte doesn’t degrade the battery’s electrodes, which helps the battery last longer. In lab experiments, Sun and colleagues were able to drain and recharge a new zinc-air battery cell 320 times over 160 hours.
Lithium-air breathing batteries
Many companies and researchers are experimenting with new lithium-air batteries that are smaller, lighter, and more energy-efficient than their forebears. Scientists from the Massachusetts Institute of Technology, the Argonne National Laboratory, and Peking University in China revealed a promising new version of lithium-air batteries. Their new device, the scientists said, can serve as a drop-in replacement for lithium-ion, while storing over five times more energy as today’s batteries.
According to the scientists, the new design uses solid oxygen electrodes to overcome many of lithium-air’s drawbacks. This new battery loses much less energy in the form of heat than earlier versions. The result is that it lasts longer and is more energy-efficient, making it a better option for electric cars and renewable energy storage.“This means faster charging for cars, as heat removal from the battery pack is less of a safety concern, as well as energy efficiency benefits,” said Ju Li, an MIT professor of nuclear science and engineering and author of the research.
Until now, lithium-air batteries inhaled outside air—driving a chemical reaction with the battery’s lithium—while electric current flows out. This oxygen is released to the atmosphere during the charging cycle. The chemical reaction produces other molecules, known as lithium peroxide, that slowly clog the battery electrodes. That raises several problems. According to Li, the solid particles formed by the reaction cause the battery to degrade faster than lithium-ion batteries, which are completely sealed from outside air. When the battery degrades, it stores less energy.
The battery is also prone to losing energy in the form of heat. Its output is more than 1.2 volts lower than the voltage needed to charge it, causing it to lose 30% of the electricity as heat. Because of that, the battery can “actually burn if you charge it too fast,” said Li. Overcharging can lead to structural damage or an explosive reaction known as thermal runaway. The new design solves these problems by closing off the battery to outside oxygen. The same electrochemical reactions take place between lithium and oxygen during charging and discharging, but they take place without ever using oxygen gas. Instead, the oxygen stays inside the battery and switches between three solid chemical compounds: Li2O, Li2O2, and LiO2. This prevents the damaging particles from forming.
The new battery sharply cuts voltage loss, so only 8% of the electrical energy is lost as heat. It also inherently guards against overcharging: The device can shift between different lithium compounds if it is being overcharged to stop activity that might cause damage. The scientists overcharged the battery to 100 times its capacity for 15 days without any damage. They also found that through 120 charging cycles, the battery only lost 2% of its capacity. The lithium-air battery can also do without components to pump air inside and out of the battery. Without these auxiliary parts, it can easily be adapted to existing devices or battery packs inside cars and power grid storage.
Aluminum Air battery
In January 2015, Japanese company Fuji Pigment Co. Ltd. announced it was developing a new type of battery called an aluminum-air battery. It simply needs to be filled with saltwater or fresh water to charge, and its theoretical specific energy level is 8,100 Wh/kg (watt hour/kilogram). Compare it with commercial lithium-ion batteries, which have specific energy levels within 100-200 Wh/kg.It should last a hefty 14 days, according to its creators Fuji Pigment. Aluminium-air batteries have a theoretical capacity more than 40 times greater than the lithium-ion cells. An aluminum-air battery generates electricity from the reaction of oxygen and aluminum, using water as an electrolyte. Furthermore, aluminum is abundant, commercially cheap and the most recycled metal in the world. As a result, aluminum-air batteries will be cheap.
A standard aluminium-air reaction consumes the aluminum anode, which must be physically replaced rather than electrically recharged. But Fuji Pigment claims that, by adding strategically placed layers of ceramic and carbon, it has managed to suppress corrosion and reaction by products, creating an aluminium-air battery that can be recharged multiple times by simply adding water. Phinergy’s has created aluminum-air battery breakthrough by using a silver-based catalyst that only allows oxygen from the ambient air into the positive cathode. The O2 then combines with the liquid electrolyte, releasing the latent electrical energy stored within the aluminium anode. The ‘air cathode’, acts like the breathable fabric, letting in O2 but no carbon dioxide, which would foul the chemical reaction.
Lighter, more compact, with greater energy output and conceivably less than half the price of lithium-ion batteries, aluminum-air technology might transform EV appeal. Renault is the front-runner to adopt this game-changing aluminium-air battery, which could yield a sevenfold boost in the electric Zoe’s 130-mile range.
Ultrafast charging titanium dioxide anode battery
Researchers at Nangyang Technological University have developed a fast-charging titanium dioxide anode battery. The batteries can be recharged up to 70 per cent in only two minutes and also have a long lifespan of over 20 years, more than 10 times compared to existing lithium-ion batteries. The titanium dioxide nanotubes were used as a gel that transfers electrons more efficiently than today’s graphite anodes, hence speeding up the charging process. It also delays deterioration, thereby multiplying the battery’s lifespan by six to seven times. The titanium dioxide batteries are not just great for smartphones, but for electric cars as well.
Another plus point for this new breed of batteries is the abundance of titanium dioxide in nature. It is a naturally occurring oxide found mainly in ilmenite and rutile ores, and many miners are dedicated to producing these minerals. One such promising mining company is White Mountain Titanium Corporation (OTCQB: WMTM), which operates its Cerro Blanco titanium project at Chile’s Atacama region. It is expected to produce as much as 112 million high-grade rutile tons, which can be used for the development of titanium dioxide anodes that could upgrade the lithium-ion batteries and finally help them keep up with the fast-changing times. Manufacturing this new nanotube gel is very easy. Titanium dioxide and sodium hydroxide are mixed together and stirred under a certain temperature so battery manufacturers will find it easy to integrate the new gel into their current production processes.
Novonix looks to commercialise ‘breakthrough’ cathode manufacturing technology
Lithium-ion battery technology developer Novonix (ASX: NVX) has developed a fully-patented “breakthrough” cathode manufacturing method using its proprietary dry particle microgranulation (DPMG) process. According to Novonix, single crystal cathode materials have become an aspiration for the lithium-ion battery industry because they outperform traditional cathode material. Compared to tradition material, the single crystal cathode design possesses enhanced energy density and ultra-long life when used in electric vehicles and energy storage systems. The research was carried out in partnership with Professor Mark Obrovac’s research group at Dalhousie University in Nova Scotia.
Novonix managing director Phil St Baker said the DPMG technique that has enabled development of the new single crystal cathode materials was a “breakthrough step-change” in improving the cost, performance and sustainability of a lithium-ion battery for electric vehicle and renewable energy applications. “The single crystal cathode development complements Novonix’s PUREgraphite anode product – both addressing the ultra-long-life battery performance requirements critical to achieving the million-mile battery life being sought by leading electric vehicle manufacturers,” Mr St Baker added.
Novonix pointed out that renowned lithium-ion battery innovator Prof Jeff Dahn, who is also based at Dalhousie University, had undertaken research into the significance of single crystal cathode with his team. Prof Dahn and his team published a paper claiming batteries with the single crystal cathode could power and electric vehicle fore more than 1.6 million kilometres and last at least two decades in grid energy storage.
Novonix BTS chief executive officer Dr Chris Burns said the research indicates the commercial opportunity in the DPMG and the single crystal cathode material. “After discovering the possibility to dry-synthesise cathode particles with polycrystalline structures using DPMG, the team immediately thought about how to use this technique to also make single crystal particles,” Dr Burns explained. “At Novonix BTS we work with various nickel manganese cobalt and nickel cobalt aluminium style cathodes and continue to find that single crystal materials are the best choice for long cycle life applications such as electric vehicles and energy storage systems,” he added. Meanwhile, Prof Obrovac claims current research has only “tapped a fraction” of the opportunities in developing advanced battery manufacturing methods and materials, while lowering costs and generating higher-performance batteries.
3D-printed lithium-ion batteries
Until now, manufacturers have had to design their devices around the size and shape of commercially available batteries. But researchers have developed a new method to 3D print lithium-ion batteries in virtually any shape.
Most lithium-ion batteries on the market come in cylindrical or rectangular shapes. Therefore, when a manufacturer is designing a product — such as a cell phone — they must dedicate a certain size and shape to the battery, which could waste space and limit design options. Theoretically, 3D-printing technologies can fabricate an entire device, including the battery and structural and electronic components, in almost any shape. However, the polymers used for 3D printing, such as poly(lactic acid) (PLA), are not ionic conductors, creating a major hurdle for printing batteries. Christopher Reyes, Benjamin Wiley and colleagues wanted to develop a process to print complete lithium-ion batteries with an inexpensive 3D printer.
The researchers increased the ionic conductivity of PLA by infusing it with an electrolyte solution. In addition, they boosted the battery’s electrical conductivity by incorporating graphene or multi-walled carbon nanotubes into the anode or cathode, respectively. To demonstrate the battery’s potential, the team 3D printed an LED bangle bracelet with an integrated lithium-ion battery. The bangle battery could power a green LED for about 60 seconds. According to the researchers, the capacity of the first-generation 3D-printed battery is about two orders of magnitude lower than that of commercial batteries, which is too low for practical use. However, they say that they have several ideas for increasing the capacity, such as replacing the PLA-based materials with 3D-printable pastes.
Market Growth
The global lithium-ion battery market is expected to grow at a CAGR of 16.4% from 2020 to 2025, reaching USD 94.4 billion by 2025 from USD 44.2 billion in 2020. Booming market for electrical vehicles, rapid technological advancements, and increasing demand for smart devices are some of the factors fueling growth in global lithium-ion battery market.Additionally, encouraging government regulations to utilize batteries in order to reduce pollution along with growing adoption in various industries are further likely to propel demand for lithium-ion batteries. However, safety concerns regarding overheating and lack of charging stations are restricting the growth of the market.
Sustained demand from developed nations in North America and Europe and expanding Asian markets such as China, India, and South Korea are driving the sales of consumer electronics. Moreover, safety concerns related to lithium-ion batteries, such as instances of explosion and fire, are pushing the manufacturers to develop safer batteries with high energy density. Therefore, the increased consumer requirements and the other factors above have created a significant opportunity for the growth of the lithium-ion battery market. The lithium-ion battery industry in APAC is expected to grow at the highest CAGR from 2020 to 2025. The automotive, consumer electronics, and power industry applications are expected to drive the demand for advanced batteries in the region. Countries such as China, the Netherlands, and Germany have implemented many initiatives and are setting strict regulations to support the growth of the electric vehicles market, which in turn, is expected to support the growth of the lithium-ion batteries market.
In terms of type, global lithium-ion battery market is categorized into lithium cobalt oxide, lithium nickel manganese cobalt oxide, lithium manganese oxide, lithium iron phosphate and others. Among these, lithium cobalt oxide accounts for the largest share in the market, on the back of its superior properties over other lithium-ion battery types, such as low rate of self-discharge, high energy density, high voltage, etc.
The market for lithium nickel manganese oxide (Li-NMC) is estimated to grow at a higher CAGR during the forecast period. The automotive industry has dominated the lithium-ion battery market. Electric vehicles require high capacity and high power that can only be provided by the use of the NMC battery type. The use of new electrolytes and additives support the charging of cell up to 4.4 V/cell. The NMC cell is growing in its range as three components involved are easy to blend and be made useful for a wide range of applications from the automotive industry to the energy storage system (ESS).
BYD Company (China), LG Chem (South Korea), Panasonic (Japan), Samsung SDI (South Korea), BAK Group (China), GS Yuasa (Japan), Hitachi (Japan), Johnson Controls (Ireland), and Toshiba (Japan) are a few leading players in the lithium-ion battery market.
References and Resources also include:
https://smallcaps.com.au/novonix-commercialise-breakthrough-cathode-manufacturing-technology/
 International Defense Security & Technology Your trusted Source for News, Research and Analysis
International Defense Security & Technology Your trusted Source for News, Research and Analysis